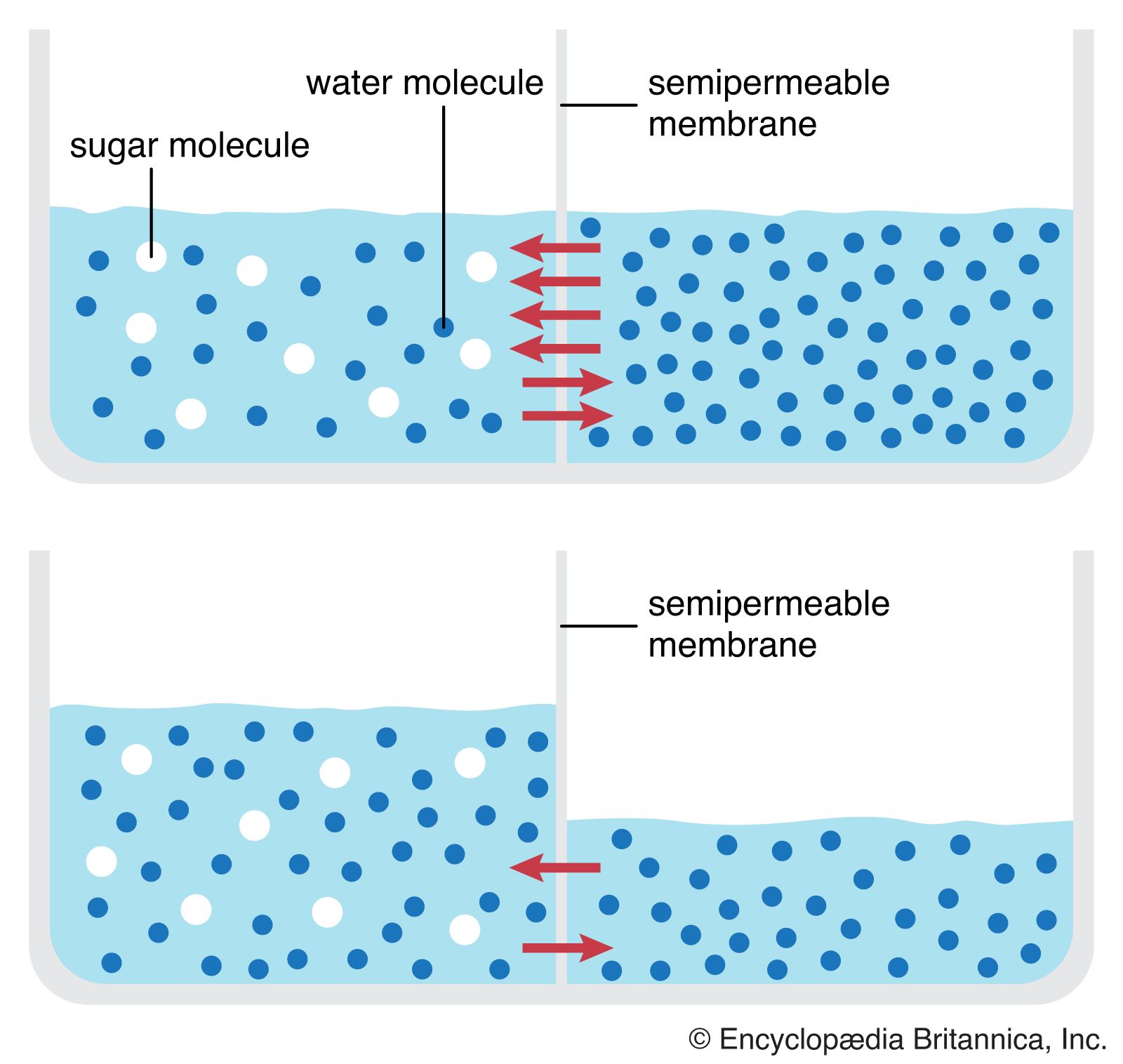
Osmosis is a fundamental biological process that plays a crucial role in maintaining the balance of fluids within living organisms. It is a type of passive transport that allows water molecules to move through a selectively permeable membrane from an area of low solute concentration to an area of high solute concentration. This movement of water molecules is driven by the concentration gradient, where water molecules tend to move from an area of higher concentration to an area of lower concentration. In the context of osmosis, the direction of water movement is critical, and it is essential to understand that osmosis occurs from low solute areas to high solute areas, rather than the other way around.
The concept of osmosis is often misunderstood, with some individuals believing that it occurs from high solute areas to low solute areas. However, this is not accurate. The movement of water molecules through a selectively permeable membrane is driven by the desire to equalize the concentration of solutes on both sides of the membrane. When there is a high concentration of solutes on one side of the membrane, water molecules will move from the side with low solute concentration to the side with high solute concentration, attempting to dilute the solutes and achieve equilibrium. This process is essential for maintaining proper cellular function, as it helps regulate the balance of fluids and electrolytes within cells.
Key Points
- Osmosis occurs from low solute areas to high solute areas, driven by the concentration gradient.
- The movement of water molecules through a selectively permeable membrane is passive, requiring no energy input.
- Osmosis plays a critical role in maintaining proper cellular function, regulating the balance of fluids and electrolytes within cells.
- The concept of osmosis is often misunderstood, with some individuals believing it occurs in the opposite direction.
- Understanding osmosis is essential for comprehending various biological processes, including cellular transport and fluid balance.
Understanding the Mechanism of Osmosis
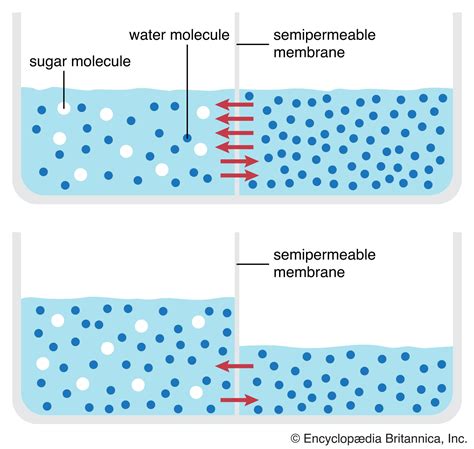
Osmosis is a complex process that involves the movement of water molecules through a selectively permeable membrane. This membrane is semi-permeable, allowing certain molecules to pass through while restricting others. The movement of water molecules through the membrane is driven by the concentration gradient, where water molecules tend to move from an area of higher concentration to an area of lower concentration. In the context of osmosis, the concentration gradient is established by the presence of solutes on one side of the membrane, which creates an area of low water concentration.
The process of osmosis can be understood by considering the structure of a selectively permeable membrane. These membranes are composed of lipid bilayers, which are impermeable to certain molecules, including ions and polar molecules. However, water molecules are able to pass through the membrane, either by diffusing through the lipid bilayer or by passing through specialized channels, such as aquaporins. The movement of water molecules through the membrane is influenced by the presence of solutes, which can either attract or repel water molecules. In general, solutes tend to attract water molecules, creating an area of low water concentration.
Factors Influencing Osmosis
Several factors can influence the rate and direction of osmosis. One of the most critical factors is the concentration gradient, which drives the movement of water molecules through the membrane. The presence of solutes on one side of the membrane creates a concentration gradient, which determines the direction of water movement. Additionally, the permeability of the membrane, the surface area of the membrane, and the temperature of the solution can all influence the rate of osmosis.
| Factor | Influence on Osmosis |
|---|---|
| Concentration Gradient | Drives the movement of water molecules through the membrane |
| Membrane Permeability | Affects the rate of water movement through the membrane |
| Surface Area | Influences the rate of osmosis by increasing the area available for water movement |
| Temperature | Affects the rate of osmosis by influencing the kinetic energy of water molecules |

Applications of Osmosis in Biology
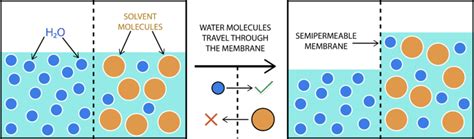
Osmosis plays a vital role in various biological processes, including cellular transport, fluid balance, and nutrient uptake. In plants, osmosis is essential for water transport, allowing water to move from the roots to the leaves through the xylem. In animals, osmosis helps regulate the balance of fluids and electrolytes within cells, maintaining proper cellular function. Additionally, osmosis is critical for the proper functioning of various organs, including the kidneys, which use osmosis to concentrate or dilute urine.
The process of osmosis is also essential for the uptake of nutrients by cells. In the small intestine, osmosis helps regulate the absorption of nutrients, including glucose and amino acids. The presence of solutes in the intestinal lumen creates a concentration gradient, which drives the movement of water molecules into the intestinal cells, facilitating the absorption of nutrients. In the kidneys, osmosis helps regulate the reabsorption of glucose and other nutrients, ensuring that essential molecules are retained in the body.
Regulation of Osmosis in the Body
The regulation of osmosis in the body is critical for maintaining proper cellular function. The body uses various mechanisms to regulate osmosis, including the production of hormones, such as antidiuretic hormone (ADH), which helps regulate water reabsorption in the kidneys. Additionally, the body uses various transport proteins, including aquaporins, to regulate the movement of water molecules through cell membranes.
The regulation of osmosis is also influenced by the presence of solutes in the body. The concentration of solutes in the blood and other bodily fluids helps regulate the movement of water molecules through cell membranes. For example, the presence of glucose in the blood can influence the movement of water molecules into cells, facilitating the uptake of glucose. Similarly, the presence of ions, such as sodium and potassium, can influence the movement of water molecules through cell membranes, helping regulate the balance of fluids and electrolytes within cells.
What is the primary driving force behind osmosis?
+The primary driving force behind osmosis is the concentration gradient, which is established by the presence of solutes on one side of the membrane.
How does osmosis occur in the body?
+Osmosis occurs in the body through the movement of water molecules through selectively permeable membranes, which are found in various cells and tissues.
What are the factors that influence osmosis?
+The factors that influence osmosis include the concentration gradient, membrane permeability, surface area, and temperature.
In conclusion, osmosis is a vital biological process that plays a critical role in maintaining the balance of fluids within living organisms. The process of osmosis occurs from low solute areas to high solute areas, driven by the concentration gradient. Understanding osmosis is essential for comprehending various biological processes, including cellular transport and fluid balance. By recognizing the importance of osmosis in maintaining proper cellular function, individuals can better appreciate the complex mechanisms that govern life.