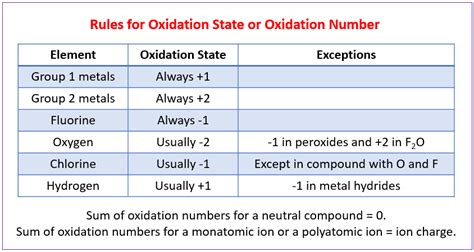
The concept of oxidation state is a fundamental aspect of chemistry, allowing us to keep track of the transfer of electrons during chemical reactions. While the rules governing oxidation states may seem complex at first, they can be simplified and understood through a systematic approach. In this article, we will delve into the world of oxidation states, exploring the key rules and principles that govern this essential concept in chemistry.
Key Points
- The oxidation state of an atom is a measure of its degree of oxidation, with higher values indicating a greater loss of electrons.
- The rules for determining oxidation states are based on the electronegativity of atoms and the type of bond they form.
- Metals tend to exhibit positive oxidation states, while nonmetals exhibit negative oxidation states.
- The oxidation state of a molecule is the sum of the oxidation states of its constituent atoms.
- Understanding oxidation states is crucial for predicting the outcome of chemical reactions and identifying the products formed.
Introduction to Oxidation State Rules
The oxidation state of an atom is defined as the hypothetical charge that the atom would have if it were to lose or gain electrons to form a bond with another atom. The rules for determining oxidation states are based on the electronegativity of atoms and the type of bond they form. By following these rules, chemists can predict the oxidation state of an atom in a particular compound or molecule.
Rule 1: The Oxidation State of a Free Element is Zero
The first rule states that the oxidation state of a free element, such as a single atom or a molecule composed of only one type of atom, is zero. This is because the atoms in a free element have not lost or gained any electrons, and therefore have no net charge. For example, the oxidation state of oxygen in O2 is zero, as each oxygen atom is bonded to another oxygen atom with a covalent bond.
Rule 2: The Oxidation State of a Monatomic Ion is Equal to its Charge
The second rule states that the oxidation state of a monatomic ion, such as Na+ or Cl-, is equal to its charge. This is because the ion has lost or gained electrons to form a stable configuration, resulting in a net charge. For example, the oxidation state of sodium in Na+ is +1, as it has lost one electron to form a stable ion.
Rule 3: The Oxidation State of Oxygen is -2, Except in Peroxides
The third rule states that the oxidation state of oxygen is -2, except in peroxides where it is -1. This is because oxygen is a highly electronegative atom that tends to attract electrons towards itself, resulting in a negative oxidation state. For example, the oxidation state of oxygen in H2O is -2, as each oxygen atom is bonded to two hydrogen atoms with covalent bonds.
| Element | Oxidation State |
|---|---|
| Oxygen (O2) | 0 |
| Oxygen (H2O) | -2 |
| Sodium (Na+) | +1 |
| Chlorine (Cl-) | -1 |
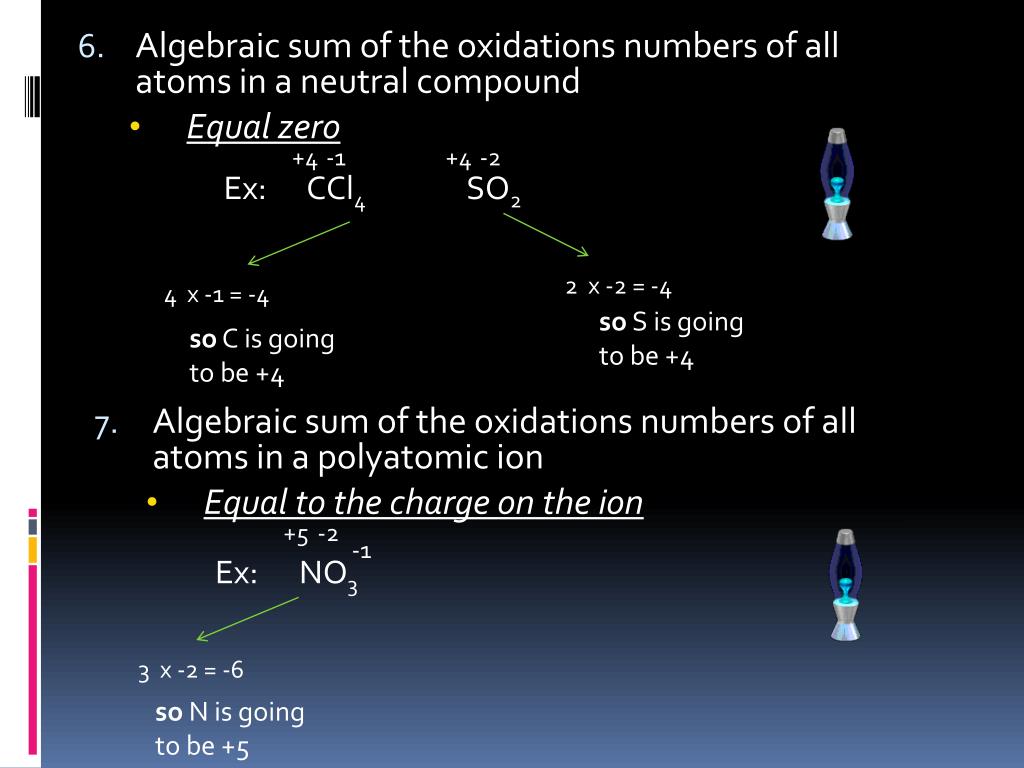
Applying Oxidation State Rules to Complex Molecules
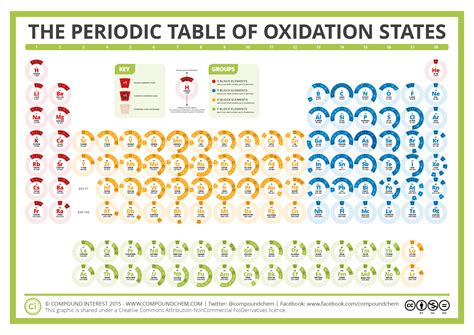
While the rules for determining oxidation states may seem straightforward, applying them to complex molecules can be challenging. By breaking down the molecule into its constituent atoms and applying the rules systematically, chemists can determine the oxidation state of each atom and predict the properties of the molecule. For example, the oxidation state of iron in Fe2O3 can be determined by applying the rules: the oxidation state of iron is +3, as each iron atom is bonded to three oxygen atoms with ionic bonds.
Rule 4: The Oxidation State of a Molecule is the Sum of the Oxidation States of its Constituent Atoms
The fourth rule states that the oxidation state of a molecule is the sum of the oxidation states of its constituent atoms. This is because the oxidation state of a molecule is a measure of its overall degree of oxidation, taking into account the oxidation states of all its constituent atoms. For example, the oxidation state of CO2 is zero, as the oxidation state of carbon is +4 and the oxidation state of each oxygen atom is -2, resulting in a net oxidation state of zero.
Rule 5: The Oxidation State of a Transition Metal can Vary
The fifth rule states that the oxidation state of a transition metal can vary, depending on the molecule or compound it is part of. This is because transition metals can exhibit multiple oxidation states, resulting in a range of possible oxidation states. For example, the oxidation state of iron can range from +2 to +6, depending on the molecule or compound it is part of.
What is the oxidation state of oxygen in a peroxide?
+The oxidation state of oxygen in a peroxide is -1, as each oxygen atom is bonded to another oxygen atom with a covalent bond.
How do you determine the oxidation state of a molecule?
+The oxidation state of a molecule is determined by applying the rules for determining oxidation states to each constituent atom and summing the resulting oxidation states.
What is the oxidation state of a free element?
+The oxidation state of a free element is zero, as the atoms in a free element have not lost or gained any electrons.
In conclusion, the rules for determining oxidation states are a fundamental concept in chemistry, allowing us to predict the properties and behavior of molecules. By understanding and applying these rules, chemists can gain valuable insights into the world of chemistry, making it an essential tool for any aspiring chemist. Whether you’re a seasoned professional or just starting out, mastering the rules of oxidation states will help you unlock the secrets of chemistry and take your understanding to the next level.