The concept of percent yield is a fundamental principle in chemistry, particularly in the realm of stoichiometry and chemical reactions. It is a measure of the efficiency of a reaction, indicating how much of the desired product is actually obtained compared to the maximum amount that could be produced based on the limiting reactant. The percent yield equation is a crucial tool in calculating this efficiency, allowing chemists to assess the success of their experiments and identify potential areas for improvement.
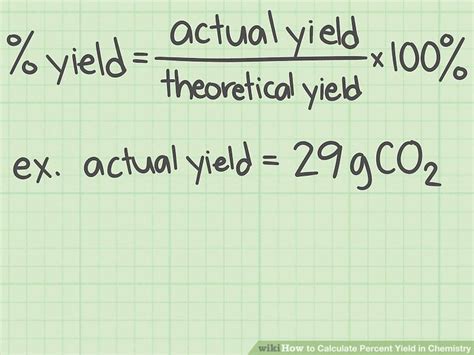
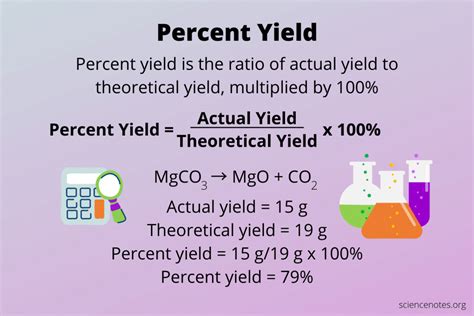
At its core, the percent yield equation is relatively straightforward. It is calculated by dividing the actual yield of a reaction (the amount of product obtained) by the theoretical yield (the maximum amount of product that could be produced), and then multiplying the result by 100 to express it as a percentage. The formula for percent yield is: Percent Yield = (Actual Yield / Theoretical Yield) * 100. This simple equation provides valuable insights into the efficiency of chemical reactions, helping chemists to refine their methods and optimize their results.
Key Points
- The percent yield equation calculates the efficiency of a chemical reaction by comparing the actual yield to the theoretical yield.
- The formula for percent yield is: Percent Yield = (Actual Yield / Theoretical Yield) * 100.
- A percent yield of 100% indicates a perfectly efficient reaction, while values below 100% suggest that some product was lost or that the reaction was incomplete.
- Percent yield is crucial in assessing the success of chemical reactions and identifying areas for improvement.
- Understanding percent yield is essential for optimizing chemical synthesis and ensuring the quality of the final product.
Understanding the Components of the Percent Yield Equation
To fully appreciate the significance of the percent yield equation, it is essential to understand its components. The actual yield refers to the amount of product that is actually obtained from a reaction, typically measured in grams or moles. The theoretical yield, on the other hand, is the maximum amount of product that could be produced based on the stoichiometry of the reaction and the amount of limiting reactant used. The limiting reactant is the reactant that is present in the smallest stoichiometric amount and thus determines the maximum amount of product that can be formed.
The calculation of theoretical yield involves understanding the mole ratios between reactants and products, as defined by the balanced chemical equation. For instance, in a reaction where 2 moles of reactant A produce 1 mole of product B, the mole ratio of A to B is 2:1. If 4 moles of A are used, theoretically, 2 moles of B could be produced, assuming the reaction goes to completion without any losses. The actual yield, however, may be less due to various factors such as incomplete reaction, side reactions, or losses during purification.
Factors Affecting Percent Yield
Several factors can influence the percent yield of a chemical reaction. These include the purity of the reactants, the reaction conditions such as temperature, pressure, and solvent choice, the efficiency of the reaction workup and purification procedures, and the presence of catalysts or inhibitors. Understanding these factors and how they impact percent yield is crucial for chemists seeking to optimize their reactions and achieve higher efficiencies.
For example, impurities in the reactants can lead to side reactions that reduce the yield of the desired product. Similarly, inappropriate reaction conditions can result in incomplete reaction or the formation of unwanted byproducts. The choice of solvent can also significantly affect the reaction rate and yield, as some solvents may facilitate the reaction while others may hinder it. Finally, the efficiency of purification procedures can greatly impact the final yield, as losses during recrystallization, distillation, or chromatography can significantly reduce the amount of product obtained.
| Factor | Impact on Percent Yield |
|---|---|
| Purity of Reactants | High purity leads to higher percent yield by minimizing side reactions. |
| Reaction Conditions | Optimized conditions (temperature, pressure, solvent) can increase percent yield by favoring the desired reaction pathway. |
| Efficiency of Purification | Effective purification minimizes product loss, thereby increasing the percent yield. |
| Catalysts or Inhibitors | Catalysts can increase the reaction rate and selectivity, potentially increasing percent yield, while inhibitors can decrease it. |
Calculating Percent Yield: A Practical Example

To illustrate the calculation of percent yield, consider a simple reaction where sodium chloride (NaCl) is produced from the reaction of sodium hydroxide (NaOH) and hydrochloric acid (HCl). The balanced equation for this reaction is: NaOH + HCl → NaCl + H2O. Suppose we start with 100 grams of NaOH and 100 grams of HCl, and after the reaction and purification, we obtain 75 grams of NaCl.
First, we need to calculate the theoretical yield of NaCl based on the limiting reactant. Assuming the molecular weights of NaOH and HCl are approximately 40 g/mol and 36.5 g/mol, respectively, and that of NaCl is about 58.5 g/mol, we can calculate the number of moles of each reactant used and then determine the limiting reactant. Let's say NaOH is the limiting reactant, and we calculate that 100 grams of NaOH would theoretically produce about 145.6 grams of NaCl (considering the mole ratio and molecular weights).
Given that the actual yield of NaCl is 75 grams, we can calculate the percent yield using the formula: Percent Yield = (Actual Yield / Theoretical Yield) * 100 = (75 g / 145.6 g) * 100 ≈ 51.5%. This means the reaction was about 51.5% efficient, indicating significant room for improvement, possibly through optimizing reaction conditions or improving the purification process.
Importance of Percent Yield in Chemical Synthesis
The concept of percent yield is not only crucial for understanding the efficiency of chemical reactions but also plays a significant role in the field of chemical synthesis. In synthesis, chemists aim to produce complex molecules through a series of reactions, and the overall efficiency of the synthesis depends on the percent yield of each individual reaction. A high percent yield in each step can significantly increase the overall yield of the final product, reducing the amount of starting material needed and minimizing waste.
Furthermore, in industrial-scale synthesis, the cost-effectiveness of a process is often closely tied to the percent yield. Higher yields can lead to lower production costs, as less material is wasted and fewer resources are required for purification and recycling. Additionally, environmental considerations favor processes with high percent yields, as they generate less waste and have a smaller environmental footprint.
What is the primary purpose of calculating percent yield in chemical reactions?
+The primary purpose of calculating percent yield is to assess the efficiency of a chemical reaction, comparing the actual amount of product obtained to the maximum theoretical amount that could be produced.
How does the purity of reactants affect the percent yield of a reaction?
+High-purity reactants can lead to higher percent yields by minimizing side reactions and ensuring that the reaction proceeds as intended, without the formation of unwanted byproducts.
What factors can influence the percent yield of a chemical reaction?
+Several factors can influence percent yield, including the reaction conditions (such as temperature and pressure), the choice of solvent, the efficiency of the purification process, and the presence of catalysts or inhibitors.
In conclusion, the percent yield equation is a fundamental tool in chemistry, allowing for the calculation of the efficiency of chemical reactions. By understanding the factors that affect percent yield and how to optimize reaction conditions and purification processes, chemists can improve the efficiency of their reactions, leading to better outcomes in both laboratory and industrial settings. The importance of percent yield extends beyond the realm of chemistry, impacting fields such as materials science, pharmacology, and environmental science, where the synthesis and application of chemicals play critical roles.