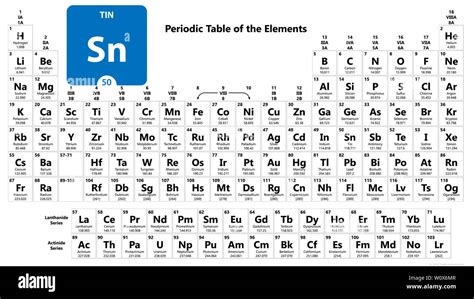
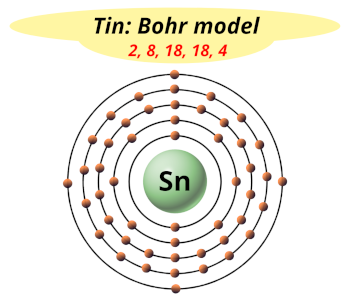
The tin element, denoted by the symbol Sn, is a chemical element with the atomic number 50 in the periodic table. It is a post-transition metal in group 14 of the periodic table, also known as the carbon group. Tin is a silvery-white, malleable, and ductile metal that is highly crystalline and has a low melting point of 231.93 °C (449.47 °F). The element's name "tin" is derived from the Old English word "tin," which was used to describe the metal.
Physical Properties of Tin

Tin has several unique physical properties that make it useful for various applications. It has a high boiling point of 2602 °C (4716 °F) and a density of 5.769 g/cm³ at 20 °C (68 °F). Tin is also highly malleable and can be molded into different shapes without breaking. Additionally, tin has a high degree of ductility, allowing it to be stretched into thin wires or sheets. The element’s crystal structure is tetragonal, with a layered arrangement of atoms that contributes to its unique properties.
Chemical Properties of Tin
Tin is a relatively inert metal, with a low reactivity that makes it resistant to corrosion. However, tin can react with certain substances, such as acids and bases, to form compounds like tin(II) chloride (SnCl₂) and tin(IV) oxide (SnO₂). The element’s chemical properties are influenced by its electronic configuration, which consists of a filled 4d subshell and a partially filled 5s subshell. This configuration allows tin to form a range of compounds with different oxidation states, including +2 and +4.
| Property | Value |
|---|---|
| Atomic Number | 50 |
| Atomic Mass | 118.71 u |
| Electron Configuration | [Kr] 4d¹⁰ 5s² 5p² |
| Melting Point | 231.93 °C (449.47 °F) |
| Boiling Point | 2602 °C (4716 °F) |
Key Points
- Tin is a post-transition metal in group 14 of the periodic table.
- The element has a low melting point of 231.93 °C (449.47 °F) and a high boiling point of 2602 °C (4716 °F).
- Tin is highly malleable and ductile, with a density of 5.769 g/cm³ at 20 °C (68 °F).
- The element's chemical properties are influenced by its electronic configuration, which consists of a filled 4d subshell and a partially filled 5s subshell.
- Tin is used in various industries, including electronics, packaging, and construction, due to its unique combination of physical and chemical properties.
Occurrence and Production of Tin
Tin is a relatively rare element in the Earth’s crust, with an abundance of approximately 2.2 parts per million (ppm). The element is primarily found in the mineral cassiterite (SnO₂), which is often associated with granite and other igneous rocks. Tin is also produced as a byproduct of the smelting of copper and other ores. The majority of tin production comes from countries such as China, Indonesia, and Peru, with the global production totaling around 290,000 metric tons in 2020.
Applications of Tin
Tin has a range of applications due to its unique properties. The element is widely used in the production of soldering alloys, which are essential for joining electronic components. Tin is also used as a coating for steel cans and other metal products, providing a protective layer against corrosion. Additionally, tin is used in the manufacture of pewter, a malleable alloy that is often used in decorative items and tableware.
In conclusion, tin is a versatile element with a unique combination of physical and chemical properties. Its high ductility and malleability make it an essential component in various industries, while its resistance to corrosion makes it an ideal coating for metal products. As research and development continue to advance, it is likely that new applications for tin will emerge, further solidifying its importance in modern technology.
What is the atomic number of tin?
+The atomic number of tin is 50.
What is the melting point of tin?
+The melting point of tin is 231.93 °C (449.47 °F).
What are the main applications of tin?
+The main applications of tin include the production of soldering alloys, coatings for steel cans and other metal products, and the manufacture of pewter.