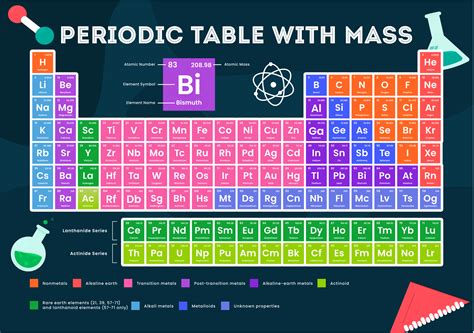
The periodic table of elements is a tabular display of the known chemical elements, organized by their atomic number (number of protons in the nucleus), electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number (number of protons in the nucleus) and are grouped into rows called periods and columns called groups or families. One of the key pieces of information provided for each element in the periodic table is its molar mass, which is the mass of one mole of the element, expressed in units of grams per mole (g/mol). The molar mass of an element is a weighted average of the masses of the naturally occurring isotopes of that element.
The molar mass of an element can be calculated by summing the masses of its protons, neutrons, and electrons, but since the mass of an electron is so small compared to the mass of a proton or neutron, it is often neglected in these calculations. The mass of a proton or neutron is approximately 1 atomic mass unit (amu), which is equal to 1.66 x 10^-24 grams. The molar mass of an element is typically calculated using the average atomic mass of the element, which takes into account the naturally occurring isotopes of the element and their relative abundances. For example, the element carbon has two naturally occurring isotopes, carbon-12 and carbon-13, with masses of 12 amu and 13 amu, respectively. The average atomic mass of carbon is 12.01 amu, which corresponds to a molar mass of 12.01 g/mol.
Key Points
- The molar mass of an element is a weighted average of the masses of its naturally occurring isotopes.
- The molar mass of an element is expressed in units of grams per mole (g/mol).
- The average atomic mass of an element is used to calculate its molar mass.
- The molar mass of an element can be used to calculate the number of moles of the element in a given sample.
- The molar mass of an element is an important piece of information in chemistry, as it is used to calculate the amounts of reactants and products in chemical reactions.
Calculation of Molar Mass

The calculation of molar mass involves summing the masses of the protons, neutrons, and electrons in an atom, but since the mass of an electron is so small, it is often neglected. The mass of a proton or neutron is approximately 1 amu, which is equal to 1.66 x 10^-24 grams. The molar mass of an element is typically calculated using the average atomic mass of the element, which takes into account the naturally occurring isotopes of the element and their relative abundances. For example, the element oxygen has three naturally occurring isotopes, oxygen-16, oxygen-17, and oxygen-18, with masses of 16 amu, 17 amu, and 18 amu, respectively. The average atomic mass of oxygen is 15.999 amu, which corresponds to a molar mass of 15.999 g/mol.
Isotopic Abundance and Molar Mass
The isotopic abundance of an element refers to the percentage of each isotope that occurs naturally in the element. The isotopic abundance of an element can affect its molar mass, as the molar mass is a weighted average of the masses of the naturally occurring isotopes. For example, the element chlorine has two naturally occurring isotopes, chlorine-35 and chlorine-37, with masses of 35 amu and 37 amu, respectively. The isotopic abundance of chlorine is approximately 75% chlorine-35 and 25% chlorine-37, which corresponds to an average atomic mass of 35.45 amu and a molar mass of 35.45 g/mol.
| Element | Atomic Mass | Molar Mass |
|---|---|---|
| Hydrogen | 1.00794 amu | 1.00794 g/mol |
| Helium | 4.002602 amu | 4.002602 g/mol |
| Oxygen | 15.9994 amu | 15.9994 g/mol |
| Carbon | 12.0107 amu | 12.0107 g/mol |
| Nitrogen | 14.0067 amu | 14.0067 g/mol |

Applications of Molar Mass

The molar mass of an element has a number of important applications in chemistry, including the calculation of the amounts of reactants and products in chemical reactions. The molar mass of an element can also be used to calculate the number of moles of the element in a given sample, which is an important piece of information in a variety of chemical calculations. For example, the molar mass of an element can be used to calculate the number of moles of the element in a given sample, which can then be used to calculate the amount of the element in grams or other units.
Chemical Reactions and Molar Mass
The molar mass of an element is an important piece of information in chemical reactions, as it is used to calculate the amounts of reactants and products. The molar mass of an element can be used to calculate the number of moles of the element in a given sample, which can then be used to calculate the amount of the element in grams or other units. For example, the reaction between hydrogen gas and oxygen gas to form water can be represented by the equation 2H2 + O2 → 2H2O. The molar mass of hydrogen is 1.00794 g/mol, and the molar mass of oxygen is 15.9994 g/mol. The molar mass of water is 18.0153 g/mol. By using the molar masses of the reactants and products, chemists can calculate the amounts of substances involved in the reaction.
In conclusion, the molar mass of an element is an important piece of information in chemistry, as it is used to calculate the amounts of reactants and products in chemical reactions. The molar mass of an element is a weighted average of the masses of the naturally occurring isotopes of the element, and it is expressed in units of grams per mole (g/mol). By understanding the isotopic abundance of an element and its effect on the molar mass, chemists can accurately calculate the amounts of substances involved in chemical reactions.
What is the molar mass of an element?
+The molar mass of an element is the mass of one mole of the element, expressed in units of grams per mole (g/mol). It is a weighted average of the masses of the naturally occurring isotopes of the element.
How is the molar mass of an element calculated?
+The molar mass of an element is calculated by summing the masses of the protons, neutrons, and electrons in an atom, but since the mass of an electron is so small, it is often neglected. The molar mass of an element is typically calculated using the average atomic mass of the element, which takes into account the naturally occurring isotopes of the element and their relative abundances.
What is the importance of molar mass in chemistry?
+The molar mass of an element is an important piece of information in chemistry, as it is used to calculate the amounts of reactants and products in chemical reactions. The molar mass of an element can also be used to calculate the number of moles of the element in a given sample, which is an important piece of information in a variety of chemical calculations.