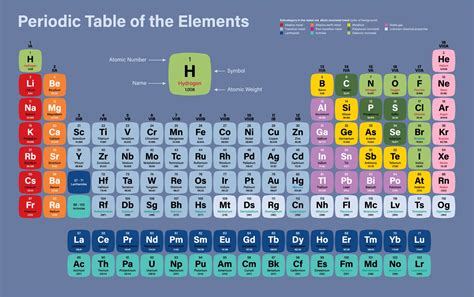
The concept of molar mass is fundamental in chemistry, as it allows us to understand the relationship between the mass of a substance and the number of particles it contains. Molar mass is defined as the mass of one mole of a substance, which is equivalent to 6.022 x 10^23 particles (Avogadro's number). In this article, we will explore the molar masses of five common substances: water (H2O), carbon dioxide (CO2), sodium chloride (NaCl), glucose (C6H12O6), and ammonia (NH3).
Molar Mass Calculations
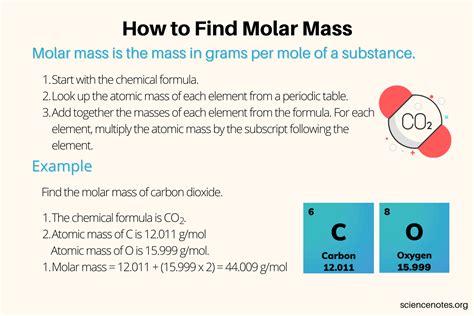
To calculate the molar mass of a substance, we need to know the atomic masses of its constituent elements. The atomic masses of the elements are typically listed on the periodic table. For example, the atomic mass of hydrogen (H) is 1.008 g/mol, oxygen (O) is 16.00 g/mol, carbon © is 12.01 g/mol, sodium (Na) is 22.99 g/mol, chlorine (Cl) is 35.45 g/mol, and nitrogen (N) is 14.01 g/mol.
Water (H2O) Molar Mass
The molar mass of water can be calculated as follows: (2 x 1.008 g/mol) + 16.00 g/mol = 18.02 g/mol. This means that one mole of water has a mass of 18.02 grams.
Carbon Dioxide (CO2) Molar Mass
The molar mass of carbon dioxide can be calculated as follows: 12.01 g/mol + (2 x 16.00 g/mol) = 44.01 g/mol. This means that one mole of carbon dioxide has a mass of 44.01 grams.
Sodium Chloride (NaCl) Molar Mass
The molar mass of sodium chloride can be calculated as follows: 22.99 g/mol + 35.45 g/mol = 58.44 g/mol. This means that one mole of sodium chloride has a mass of 58.44 grams.
Glucose (C6H12O6) Molar Mass
The molar mass of glucose can be calculated as follows: (6 x 12.01 g/mol) + (12 x 1.008 g/mol) + (6 x 16.00 g/mol) = 180.16 g/mol. This means that one mole of glucose has a mass of 180.16 grams.
Ammonia (NH3) Molar Mass
The molar mass of ammonia can be calculated as follows: 14.01 g/mol + (3 x 1.008 g/mol) = 17.03 g/mol. This means that one mole of ammonia has a mass of 17.03 grams.
Key Points
- The molar mass of a substance is the mass of one mole of that substance.
- The atomic masses of the elements are used to calculate the molar mass of a substance.
- The molar mass of water (H2O) is 18.02 g/mol.
- The molar mass of carbon dioxide (CO2) is 44.01 g/mol.
- The molar mass of sodium chloride (NaCl) is 58.44 g/mol.
- The molar mass of glucose (C6H12O6) is 180.16 g/mol.
- The molar mass of ammonia (NH3) is 17.03 g/mol.
| Substance | Molar Mass (g/mol) |
|---|---|
| Water (H2O) | 18.02 |
| Carbon Dioxide (CO2) | 44.01 |
| Sodium Chloride (NaCl) | 58.44 |
| Glucose (C6H12O6) | 180.16 |
| Ammonia (NH3) | 17.03 |

In conclusion, the molar masses of the five substances discussed in this article are: water (H2O) = 18.02 g/mol, carbon dioxide (CO2) = 44.01 g/mol, sodium chloride (NaCl) = 58.44 g/mol, glucose (C6H12O6) = 180.16 g/mol, and ammonia (NH3) = 17.03 g/mol. These values are essential in chemistry and are used in various chemical calculations and applications.
What is the definition of molar mass?
+Molar mass is the mass of one mole of a substance, which is equivalent to 6.022 x 10^23 particles (Avogadro’s number).
How is the molar mass of a substance calculated?
+The molar mass of a substance is calculated by summing the atomic masses of its constituent elements.
What is the molar mass of water (H2O)?
+The molar mass of water (H2O) is 18.02 g/mol.
What is the molar mass of carbon dioxide (CO2)?
+The molar mass of carbon dioxide (CO2) is 44.01 g/mol.
What is the molar mass of sodium chloride (NaCl)?
+The molar mass of sodium chloride (NaCl) is 58.44 g/mol.
What is the molar mass of glucose (C6H12O6)?
+The molar mass of glucose (C6H12O6) is 180.16 g/mol.
What is the molar mass of ammonia (NH3)?
+The molar mass of ammonia (NH3) is 17.03 g/mol.