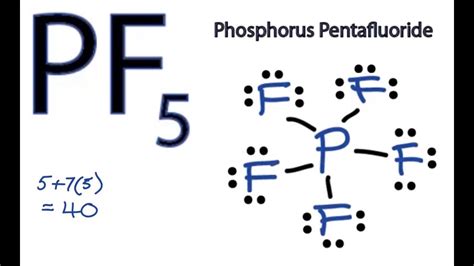
The PF5 Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding of phosphorus pentafluoride. To understand this structure, it's essential to have a solid grasp of basic chemistry principles, including valence shell electron pair repulsion (VSEPR) theory and the octet rule. Phosphorus pentafluoride, with the chemical formula PF5, consists of one phosphorus atom bonded to five fluorine atoms. This molecule is of particular interest due to its unique properties and applications in various chemical reactions.
Constructing the Lewis structure for PF5 involves several steps. First, determine the total number of valence electrons available for bonding. Phosphorus, being in group 15 of the periodic table, has 5 valence electrons, and each fluorine atom, found in group 17, contributes 7 valence electrons. Thus, the total valence electrons for PF5 are calculated as 5 (from phosphorus) + 5*7 (from five fluorine atoms) = 5 + 35 = 40 electrons. However, since the molecule is neutral, we only consider the valence electrons, which are the electrons in the outermost shell of each atom.
Key Points
- The PF5 molecule has a trigonal bipyramidal geometry due to the arrangement of its electron pairs.
- Phosphorus acts as the central atom, with five fluorine atoms bonded to it.
- The Lewis structure is crucial for understanding the chemical properties and reactivity of PF5.
- The molecule's stability is attributed to the fulfillment of the octet rule for all atoms, except phosphorus, which expands its octet.
- Understanding PF5's structure is vital for its applications in chemical synthesis and as a reagent in various reactions.
Understanding the Lewis Structure
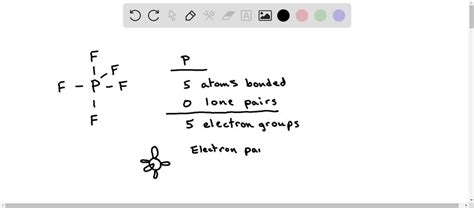
The Lewis structure of PF5 can be drawn by first placing the phosphorus atom at the center, as it can form more bonds than fluorine. Then, arrange the five fluorine atoms around the phosphorus, ensuring each fluorine atom shares one pair of electrons with phosphorus to form a covalent bond. Since phosphorus has five valence electrons and each fluorine atom contributes one electron to the bond, this results in five single bonds. Each fluorine atom then has three lone pairs of electrons, fulfilling the octet rule for fluorine. Phosphorus, however, ends up with ten valence electrons, expanding its octet, which is stable due to the low electronegativity of phosphorus compared to fluorine and the ability of phosphorus to expand its octet due to the availability of d-orbitals.
Geometry and Polarity
The geometry of PF5 is determined by the arrangement of its electron groups (bonding and non-bonding pairs) around the central phosphorus atom. According to VSEPR theory, five electron groups (the five P-F bonds) around a central atom will arrange themselves in a trigonal bipyramidal geometry to minimize repulsions. This geometry has three equatorial positions and two axial positions. The axial bonds are slightly longer than the equatorial bonds due to the greater repulsion from the equatorial bonds. PF5 is a non-polar molecule because the dipole moments of the P-F bonds cancel each other out due to the symmetrical trigonal bipyramidal arrangement.
| Property | Value/Description |
|---|---|
| Molecular Formula | PF5 |
| Molecular Geometry | Trigonal Bipyramidal |
| Polarity | Non-polar |
| Central Atom | Phosphorus |
| Number of Electron Groups | 5 |
Applications and Safety Considerations

PF5 has several applications in chemical synthesis, including the preparation of other phosphorus compounds and as a fluorinating agent. However, it’s crucial to handle PF5 with care due to its toxicity and reactivity. PF5 is highly corrosive and can cause severe burns upon contact with skin. It also reacts violently with water and moisture, producing toxic fumes. Thus, handling PF5 requires appropriate protective gear and equipment, and it should be stored in a cool, dry place, away from incompatible substances.
Synthesis and Reactions
Phosphorus pentafluoride can be synthesized through the reaction of phosphorus trifluoride (PF3) with fluorine gas. This reaction is highly exothermic and requires careful control of conditions to produce PF5 efficiently and safely. PF5 can also undergo various reactions, including hydrolysis, where it reacts with water to form phosphoric acid and hydrofluoric acid, and it can act as a Lewis acid, accepting a pair of electrons from a Lewis base.
In conclusion, understanding the PF5 Lewis structure is essential for grasping the molecular properties, reactivity, and applications of phosphorus pentafluoride. The unique trigonal bipyramidal geometry and the expanded octet of phosphorus contribute to the molecule's stability and chemical behavior. As with any chemical compound, especially those as reactive and toxic as PF5, it's vital to approach its handling and application with thorough knowledge and appropriate caution.
What is the molecular geometry of PF5?
+The molecular geometry of PF5 is trigonal bipyramidal, as determined by the arrangement of its five electron groups around the central phosphorus atom.
Why is PF5 non-polar despite having polar P-F bonds?
+PF5 is non-polar because the dipole moments of the P-F bonds cancel each other out due to the symmetrical trigonal bipyramidal arrangement of the molecule.
What are some safety considerations when handling PF5?
+PF5 is highly corrosive and toxic. It requires careful handling, including the use of protective gear, and should be stored in a cool, dry place away from incompatible substances.