The PH3 Lewis structure is a fundamental concept in chemistry, representing the phosphine molecule. Understanding how to draw and interpret this structure is crucial for chemists and students alike. Here are five key tips to help master the PH3 Lewis structure:
Understanding the Basics of Lewis Structures
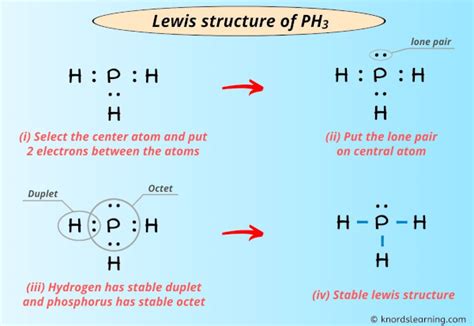
A Lewis structure is a two-dimensional representation of a molecule, showing how electrons are arranged in the molecule. It’s essential to understand the basic rules of drawing Lewis structures, including counting valence electrons, determining the central atom, and drawing single bonds between atoms. For PH3, phosphorus (P) is the central atom, and hydrogen (H) atoms surround it.
Counting Valence Electrons
To draw the PH3 Lewis structure, start by counting the valence electrons. Phosphorus has 5 valence electrons, and each hydrogen atom has 1 valence electron. Since there are 3 hydrogen atoms, the total number of valence electrons is 5 (from P) + 3 (from H) = 8. This step is critical because it determines the number of electrons available for bonding and lone pairs.
| Atom | Valence Electrons |
|---|---|
| Phosphorus (P) | 5 |
| Hydrogen (H) | 1 |
| Total | 8 |
Determining the Central Atom and Drawing Bonds
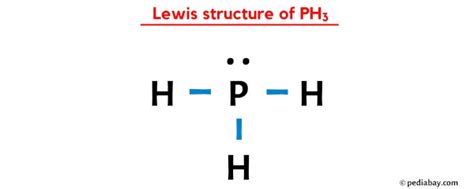
The central atom in the PH3 molecule is phosphorus. Draw single bonds between the phosphorus atom and each of the three hydrogen atoms. This step uses 6 of the 8 valence electrons (2 electrons per bond, and there are 3 bonds). The remaining 2 electrons are placed on the phosphorus atom as a lone pair, satisfying the octet rule for phosphorus and ensuring each hydrogen atom has 2 electrons.
Finalizing the Lewis Structure
After drawing the bonds and placing the lone pair on the phosphorus atom, the PH3 Lewis structure is complete. It’s essential to verify that the structure follows the octet rule for all atoms (except hydrogen, which follows the duet rule) and that the total number of valence electrons is correctly accounted for.
Key Points
- Phosphorus is the central atom in the PH3 molecule.
- There are 8 valence electrons in total (5 from P and 3 from H).
- The PH3 Lewis structure has 3 single bonds between P and H, using 6 electrons.
- A lone pair of electrons is placed on the phosphorus atom to satisfy the octet rule.
- Each hydrogen atom has 2 electrons, satisfying the duet rule.
Mastering the PH3 Lewis structure is fundamental for understanding more complex molecules and chemical reactions. By following these tips and practicing, one can become proficient in drawing and interpreting Lewis structures, a crucial skill in chemistry.
What is the central atom in the PH3 molecule?
+The central atom in the PH3 molecule is phosphorus (P).
How many valence electrons are in the PH3 molecule?
+There are 8 valence electrons in the PH3 molecule (5 from phosphorus and 3 from hydrogen).
What rule does hydrogen follow in terms of electron configuration?
+Hydrogen follows the duet rule, meaning it needs 2 electrons to achieve a stable configuration.
By applying these principles and tips, one can accurately draw the PH3 Lewis structure and gain a deeper understanding of molecular chemistry. Remember, practice is key to mastering Lewis structures, so it’s essential to apply these skills to a variety of molecules.