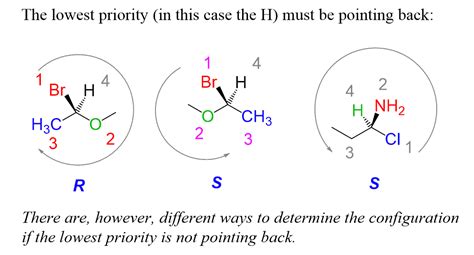
The concept of R and S configuration is a fundamental aspect of stereochemistry, a branch of chemistry that deals with the three-dimensional arrangement of atoms in molecules. Understanding R and S configuration is crucial for chemists, as it helps them predict the properties and behavior of molecules. In this article, we will delve into the world of R and S configuration, exploring its definition, importance, and application in various fields of chemistry.
Introduction to R and S Configuration
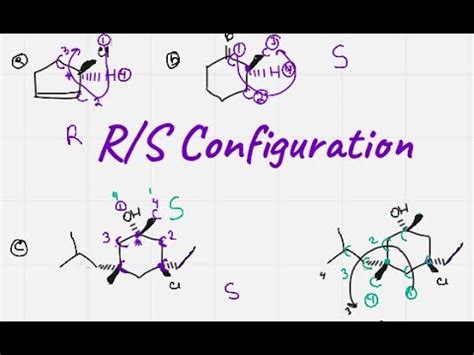
The R and S configuration system was introduced by the American chemist Robert Sidney Cahn, the British chemist Christopher Ingold, and the Scottish chemist Vladimir Prelog in the 1950s. This system provides a way to describe the stereochemistry of molecules, which is essential for understanding their properties and behavior. The R and S configuration is based on the arrangement of atoms around a stereocenter, which is a carbon atom that is bonded to four different groups.
Definition of R and S Configuration
The R and S configuration is defined by the sequence of atoms around a stereocenter. The sequence is determined by the priority of the atoms, which is based on their atomic number. The atom with the highest priority is assigned the number 1, the next highest priority is assigned the number 2, and so on. The sequence of atoms is then viewed from the side of the stereocenter that is opposite to the lowest priority atom. If the sequence of atoms is clockwise, the configuration is R (from the Latin “rectus,” meaning “right”); if the sequence is counterclockwise, the configuration is S (from the Latin “sinister,” meaning “left”).
| Atom Priority | Atomic Number |
|---|---|
| 1 | Higher atomic number |
| 2 | Lower atomic number |
| 3 | Lower atomic number |
| 4 | Lowest atomic number |
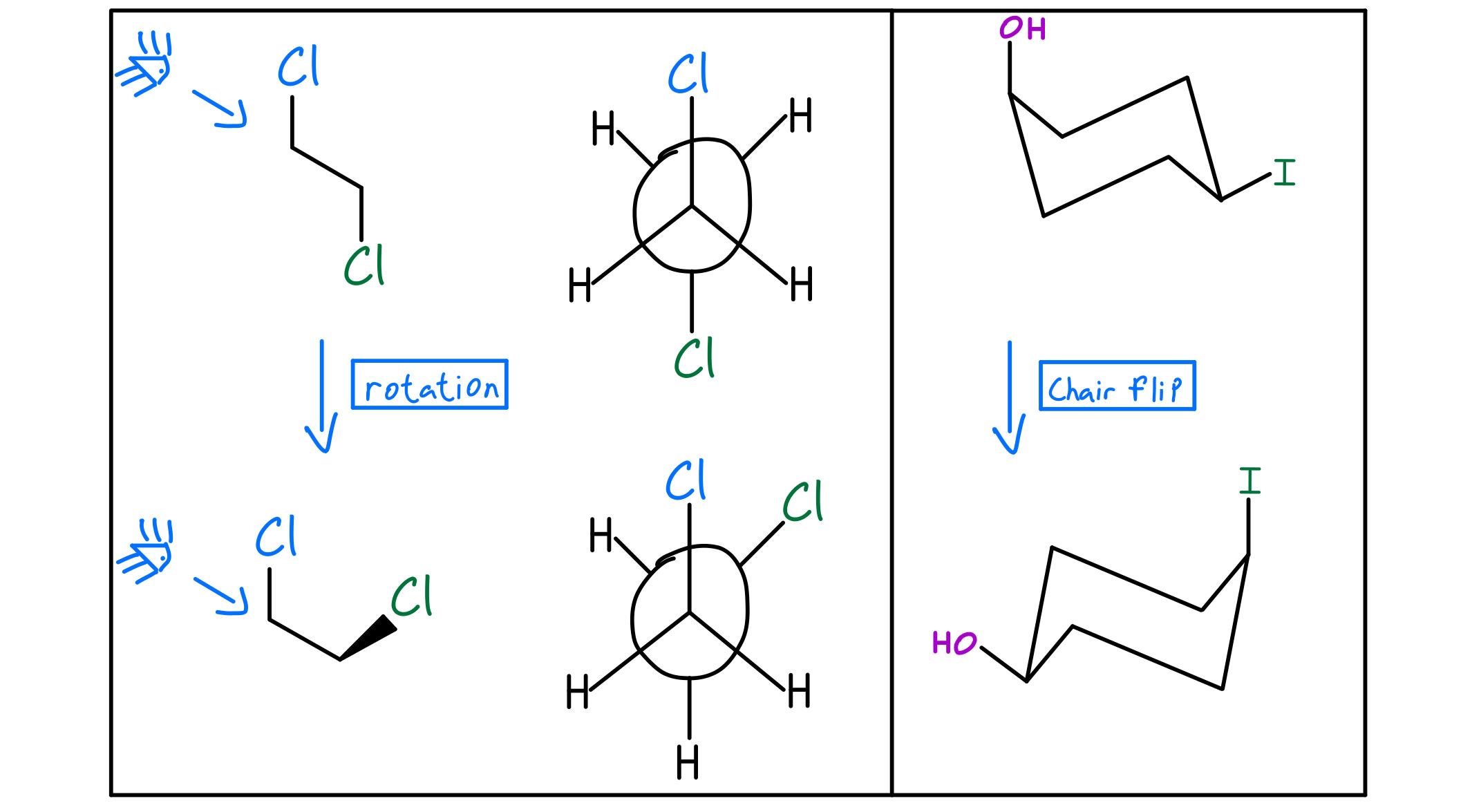
Importance of R and S Configuration
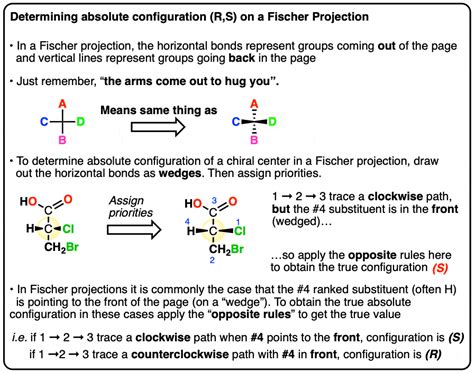
The R and S configuration is crucial in understanding the properties and behavior of molecules. Many biological molecules, such as amino acids and sugars, have specific R and S configurations that are essential for their function. The R and S configuration also plays a critical role in the development of pharmaceuticals, as many drugs are chiral molecules that require specific R and S configurations to be effective.
Application of R and S Configuration
The R and S configuration has numerous applications in various fields of chemistry, including organic synthesis, pharmacology, and biochemistry. Understanding the R and S configuration is essential for synthesizing chiral molecules, which are molecules that have a specific three-dimensional arrangement of atoms. The R and S configuration is also crucial in the development of pharmaceuticals, as many drugs are chiral molecules that require specific R and S configurations to be effective.
Key Points
- The R and S configuration is a fundamental concept in stereochemistry that describes the three-dimensional arrangement of atoms in molecules.
- The R and S configuration is essential for understanding the properties and behavior of molecules, particularly chiral molecules.
- The R and S configuration has numerous applications in various fields of chemistry, including organic synthesis, pharmacology, and biochemistry.
- Understanding the R and S configuration is crucial for synthesizing chiral molecules and developing effective pharmaceuticals.
- The R and S configuration is not a fixed property of a molecule, but rather a way to describe its stereochemistry.
Conclusion
In conclusion, the R and S configuration is a fundamental concept in stereochemistry that is essential for understanding the properties and behavior of molecules. The R and S configuration has numerous applications in various fields of chemistry, including organic synthesis, pharmacology, and biochemistry. By understanding the R and S configuration, chemists can synthesize chiral molecules and develop effective pharmaceuticals. As research in stereochemistry continues to evolve, the importance of the R and S configuration will only continue to grow.
What is the difference between R and S configuration?
+The R and S configuration refers to the arrangement of atoms around a stereocenter. The R configuration is assigned when the sequence of atoms is clockwise, while the S configuration is assigned when the sequence is counterclockwise.
Why is the R and S configuration important in chemistry?
+The R and S configuration is important in chemistry because it helps predict the properties and behavior of molecules. Many biological molecules, such as amino acids and sugars, have specific R and S configurations that are essential for their function.
How is the R and S configuration used in pharmaceuticals?
+The R and S configuration is crucial in the development of pharmaceuticals, as many drugs are chiral molecules that require specific R and S configurations to be effective. Understanding the R and S configuration helps chemists synthesize chiral molecules and develop effective pharmaceuticals.
Meta description: Learn about the R and S configuration, a fundamental concept in stereochemistry that describes the three-dimensional arrangement of atoms in molecules. Understand its importance and application in various fields of chemistry.