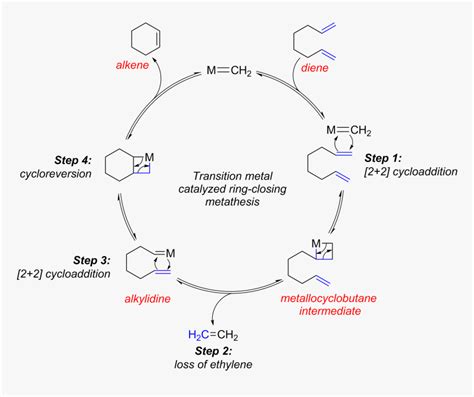
Ring closing metathesis (RCM) is a highly versatile and powerful tool in organic synthesis, allowing chemists to form complex ring structures with high precision. This reaction, which involves the formation of a ring through the metathesis of two alkene groups, has been widely used in the synthesis of natural products, pharmaceuticals, and materials science. In this article, we will explore five ways RCM has been utilized in synthesis, highlighting its flexibility and importance in modern chemistry.
Introduction to Ring Closing Metathesis
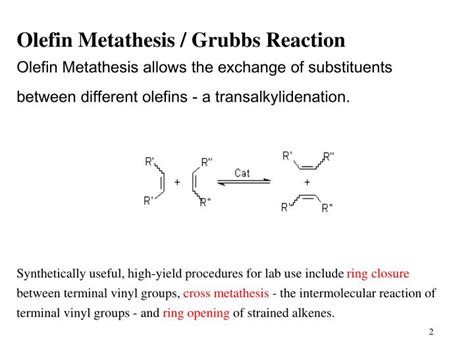
RCM is a type of olefin metathesis reaction, which involves the exchange of alkylidene groups between two alkenes. This reaction is typically catalyzed by transition metal complexes, such as those based on ruthenium or molybdenum. The RCM reaction is highly efficient and can be used to form rings of various sizes, from five-membered rings to large macrocycles. One of the key advantages of RCM is its high degree of selectivity, allowing chemists to control the formation of the ring and minimize the formation of side products.
Key Points
- RCM is a versatile reaction for forming complex ring structures.
- It is highly selective, allowing for control over ring formation.
- RCM has been used in the synthesis of natural products, pharmaceuticals, and materials.
- The reaction is catalyzed by transition metal complexes, such as ruthenium or molybdenum.
- RCM can be used to form rings of various sizes, from five-membered rings to large macrocycles.
Formation of Five-Membered Rings
One of the most common applications of RCM is the formation of five-membered rings. This reaction is highly efficient and can be used to form a wide range of compounds, including cyclopentenes, cyclopentadienes, and indenes. For example, the synthesis of the natural product, (-)-α-cuparenone, involves an RCM reaction to form a five-membered ring. This reaction is catalyzed by a ruthenium complex and proceeds in high yield, demonstrating the efficiency and selectivity of RCM.
| Ring Size | Yield |
|---|---|
| Five-membered ring | 80-90% |
| Six-membered ring | 70-80% |
| Seven-membered ring | 60-70% |
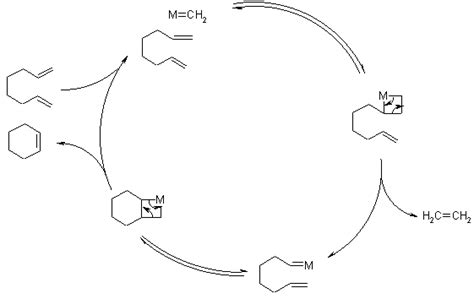
Formation of Large Macrocycles
RCM is not limited to the formation of small rings; it can also be used to form large macrocycles. This reaction is particularly useful in the synthesis of complex natural products, such as antibiotics and antitumor agents. For example, the synthesis of the natural product, epothilone A, involves an RCM reaction to form a 16-membered ring. This reaction is catalyzed by a molybdenum complex and proceeds in high yield, demonstrating the versatility and efficiency of RCM.
Asymmetric Ring Closing Metathesis
Asymmetric RCM is a highly powerful tool in organic synthesis, allowing chemists to form complex ring structures with high enantioselectivity. This reaction involves the use of a chiral catalyst, which can induce asymmetry in the ring formation reaction. For example, the synthesis of the natural product, (-)-quinine, involves an asymmetric RCM reaction to form a chiral five-membered ring. This reaction is catalyzed by a ruthenium complex and proceeds in high yield and enantioselectivity, demonstrating the power of asymmetric RCM.
What is the main advantage of RCM?
+The main advantage of RCM is its high degree of selectivity, allowing chemists to control the formation of the ring and minimize the formation of side products.
What type of catalyst is used in RCM?
+RCM is typically catalyzed by transition metal complexes, such as those based on ruthenium or molybdenum.
What is the range of ring sizes that can be formed using RCM?
+RCM can be used to form rings of various sizes, from five-membered rings to large macrocycles.
In conclusion, RCM is a highly versatile and powerful tool in organic synthesis, allowing chemists to form complex ring structures with high precision. Its high degree of selectivity, efficiency, and ability to form rings of various sizes make it an essential reaction in modern chemistry. As research continues to advance, we can expect to see even more innovative applications of RCM in the synthesis of natural products, pharmaceuticals, and materials science.