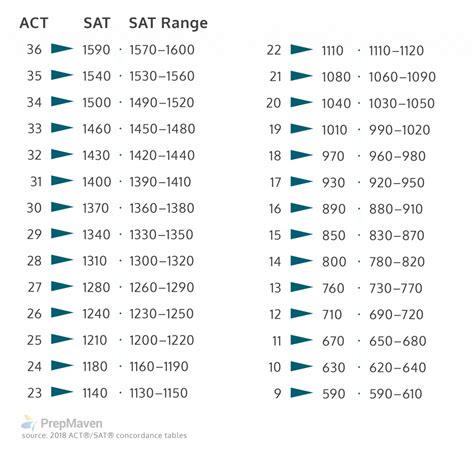
The element Tin, denoted by the symbol Sn, has an atomic number of 50. This means that a tin atom has 50 protons in its atomic nucleus. As a post-transition metal, tin is located in the p-block of the periodic table, in group 14, and is the fourth element of the sixth period. Its atomic number is a fundamental property that distinguishes it from other elements and determines its position in the periodic table.
Introduction to Tin and Its Properties

Tin, with its atomic number of 50, exhibits a range of unique properties that make it valuable for various industrial and technological applications. Its electron configuration is [Kr] 4d10 5s2 5p2, which is characteristic of its position in the periodic table. The atomic mass of tin is approximately 118.71 u (unified atomic mass units), which is a weighted average of its naturally occurring isotopes. Tin is known for its malleability, ductility, and low melting point of about 231.93 °C, making it an ideal material for soldering and other uses.
Chemical Properties and Reactions of Tin
Given its atomic number and electron configuration, tin displays a variety of chemical properties. It can exist in two main oxidation states: +2 and +4, represented by its ions Sn2+ and Sn4+, respectively. Tin reacts with oxygen to form tin oxides, and it also reacts with acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), to produce tin salts. The reactivity of tin is influenced by its atomic number and the resulting electron configuration, which dictates its ability to form bonds with other elements.
| Property | Value |
|---|---|
| Atomic Number | 50 |
| Atomic Mass | 118.71 u |
| Electron Configuration | [Kr] 4d10 5s2 5p2 |
| Melting Point | 231.93 °C |
Key Points
- Tin's atomic number is 50, which defines its position in the periodic table and determines its chemical properties.
- The element has a range of unique physical properties, including malleability, ductility, and a low melting point, making it versatile for various applications.
- Tin exists in two main oxidation states (+2 and +4) and reacts with oxygen and acids to form tin oxides and salts, respectively.
- Its atomic mass is approximately 118.71 u, which is a critical factor in its chemical reactions and applications.
- Understanding the relationship between tin's atomic number, electron configuration, and its properties is essential for leveraging its potential in technology and industry.
Applications and Uses of Tin
The applications of tin are diverse, ranging from soldering in electronic devices to plating steel cans for food preservation. Its low toxicity and corrosion resistance make it an ideal material for these purposes. Furthermore, tin is used in semiconductors, as a catalyst in the production of polyethylene terephthalate (PET), and in the manufacture of pesticides and paints. The unique properties of tin, stemming from its atomic number and resulting electron configuration, underpin its utility across these various sectors.
Sustainability and Environmental Considerations
As with the extraction and use of any metal, the production of tin raises environmental and sustainability concerns. The mining of tin can lead to deforestation, water pollution, and soil degradation. Additionally, the smelting process can release harmful emissions. However, efforts are being made to improve the sustainability of tin production, including recycling and the development of more environmentally friendly extraction and refining techniques. The environmental impact of tin must be considered alongside its benefits, necessitating a balanced approach to its use and management.
What is the primary use of tin in modern technology?
+Tin is primarily used in soldering for electronic devices due to its low melting point and ability to form strong bonds between metals.
How does the atomic number of tin influence its chemical properties?
+The atomic number of 50 gives tin its unique electron configuration, which in turn dictates its ability to form bonds, react with other elements, and exhibit specific oxidation states.
What are the environmental concerns associated with tin production?
+The extraction and processing of tin can lead to environmental issues such as deforestation, water pollution, and the release of harmful emissions, highlighting the need for sustainable practices.
In conclusion, the atomic number of tin, 50, underlies its chemical and physical properties, making it a valuable element for a wide range of applications. From its use in electronics and food packaging to its role in semiconductors and as a catalyst, tin’s unique characteristics, derived from its atomic number and electron configuration, position it as a critical component in modern technology and industry. As we continue to develop and rely on tin for various purposes, it is essential to address the environmental and sustainability challenges associated with its production, ensuring that its benefits are realized while minimizing its negative impacts.