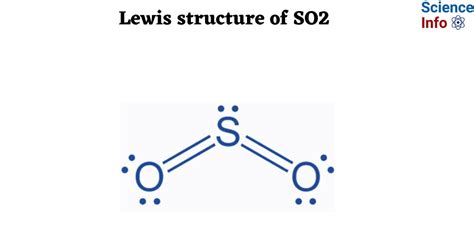
The sulfur dioxide molecule, denoted as SO2, is a crucial compound in various atmospheric and industrial processes. Understanding its Lewis dot structure is essential for grasping its chemical properties and reactivity. In this article, we will delve into the world of SO2, exploring its Lewis dot structure, electronic configuration, and the implications of its molecular geometry.
To begin with, the Lewis dot structure is a simplified representation of the molecule, focusing on the valence electrons and their distribution among the atoms. For SO2, we start with the sulfur atom (S) and the two oxygen atoms (O). Sulfur, being in group 16 of the periodic table, has 6 valence electrons, while each oxygen atom has 6 valence electrons as well. The total number of valence electrons in the SO2 molecule is 18 (6 from sulfur + 6 from each of the two oxygen atoms).
Key Points
- The SO2 molecule consists of one sulfur atom and two oxygen atoms.
- Sulfur has 6 valence electrons, and each oxygen has 6 valence electrons, totaling 18 valence electrons in the SO2 molecule.
- The Lewis dot structure of SO2 involves a double bond between sulfur and one oxygen and a single bond between sulfur and the other oxygen, with a lone pair on the sulfur.
- The molecular geometry of SO2 is bent or V-shaped due to the lone pair on the sulfur atom, which influences its chemical properties.
- Understanding the Lewis dot structure of SO2 is crucial for predicting its reactivity and behavior in various chemical reactions.
Constructing the Lewis Dot Structure
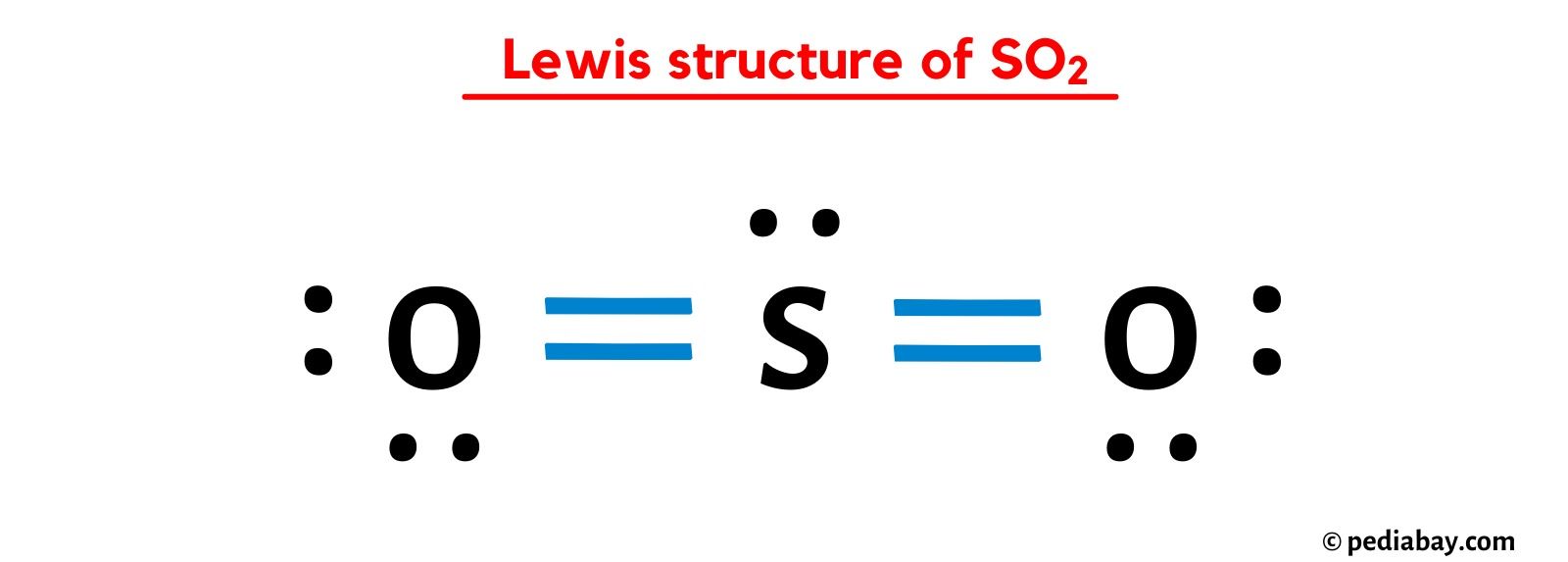
Constructing the Lewis dot structure for SO2 involves several steps. First, we place the atoms relative to each other, with the sulfur atom as the central atom due to its lower electronegativity compared to oxygen. Then, we calculate the total number of valence electrons available, which we’ve determined to be 18. Next, we draw single bonds between the sulfur and each oxygen atom, which accounts for 4 electrons. This leaves us with 14 remaining valence electrons.
After forming the single bonds, we distribute the remaining electrons as lone pairs around the atoms to satisfy the octet rule for each atom. The octet rule states that an atom tends to have 8 electrons in its outer shell to achieve stability. By adding a double bond between sulfur and one of the oxygen atoms, we satisfy the octet rule for both the sulfur and that oxygen, while the other oxygen atom has two lone pairs and a single bond to sulfur, also satisfying the octet rule for that oxygen. This configuration results in a structure where sulfur has a lone pair, a single bond to one oxygen, and a double bond to the other oxygen.
Molecular Geometry and Its Implications
The molecular geometry of SO2 is influenced by the lone pair on the sulfur atom. According to VSEPR (Valence Shell Electron Pair Repulsion) theory, electron pairs (both bonding and nonbonding) repel each other. In the case of SO2, the presence of a lone pair on sulfur, along with the two bonds (one single and one double) to the oxygen atoms, leads to a bent or V-shaped molecular geometry. This geometry is a result of the lone pair occupying more space than the bonding pairs and thus exerting greater repulsion, pushing the oxygen atoms closer together and resulting in the bent shape.
| Property | Value |
|---|---|
| Molecular Formula | SO2 |
| Molecular Weight | 64.065 g/mol |
| Boiling Point | -10°C |
| Melting Point | -72.4°C |
Relevance and Applications
The understanding of SO2’s Lewis dot structure and its implications on molecular geometry is not merely an academic exercise but has far-reaching consequences in environmental science, chemistry, and industry. SO2 is a major air pollutant, primarily resulting from the burning of fossil fuels by power plants and automobiles. Its presence in the atmosphere contributes to acid rain, which has detrimental effects on ecosystems, buildings, and human health. Moreover, SO2 plays a critical role in the production of sulfuric acid, a vital chemical in numerous industrial processes.
In conclusion, the Lewis dot structure of SO2, with its characteristic bent geometry, underpins the chemical properties and reactivity of this molecule. By grasping the fundamentals of its electronic configuration and molecular shape, we can better appreciate the complex roles SO2 plays in both natural and industrial contexts. As we move forward in addressing environmental challenges and developing new technologies, the foundational knowledge of molecular structures like that of SO2 will continue to be indispensable.
What is the significance of the lone pair in the SO2 molecule?
+The lone pair on the sulfur atom in SO2 is significant because it influences the molecular geometry, making it bent or V-shaped, and it also enables SO2 to act as a Lewis base, participating in coordinate covalent bonding.
How does the molecular geometry of SO2 affect its chemical properties?
+The bent geometry of SO2, resulting from the lone pair on sulfur, affects its chemical properties by making it more reactive and capable of forming complexes. This geometry also influences its polarity, making SO2 a polar molecule.
What are the primary sources of SO2 emissions?
+The primary sources of SO2 emissions include the burning of fossil fuels by power plants and automobiles. Industrial processes, such as the production of sulfuric acid, also contribute to SO2 emissions.