The sulfur trioxide (SO3) molecule is a fundamental concept in chemistry, and understanding its Lewis structure is crucial for grasping its properties and reactivity. The SO3 Lewis structure represents the arrangement of electrons within the molecule, providing insights into its bonding and overall molecular geometry. In this article, we will explore the different ways to draw the SO3 Lewis structure, highlighting the key principles and considerations involved in each approach.
Key Points
- The SO3 molecule has a total of 26 valence electrons, which must be distributed among its atoms to satisfy the octet rule.
- There are three primary ways to draw the SO3 Lewis structure, each with its unique characteristics and implications for the molecule's properties.
- The choice of Lewis structure can significantly impact our understanding of the molecule's reactivity, polarity, and overall behavior in different chemical environments.
- Each Lewis structure should be evaluated based on its ability to minimize formal charges, maximize bond order, and satisfy the octet rule for all atoms involved.
- The most appropriate Lewis structure for SO3 is one that balances these factors, providing a comprehensive understanding of the molecule's electronic configuration and its consequences for chemical reactivity.
Method 1: Traditional Lewis Structure with Double Bonds
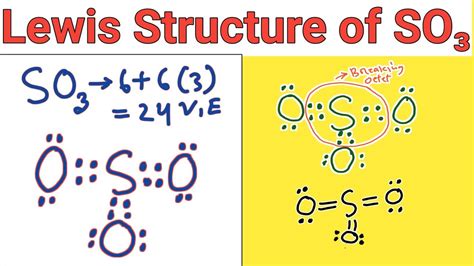
The traditional approach to drawing the SO3 Lewis structure involves creating double bonds between the sulfur atom and two of the oxygen atoms, while the third oxygen atom forms a single bond with sulfur. This structure is often the first one taught in introductory chemistry courses due to its simplicity and adherence to the octet rule for all atoms except sulfur, which expands its octet to accommodate more than eight electrons.
This method results in a structure where sulfur has two double bonds and one single bond, with formal charges assigned to minimize the overall charge on the molecule. However, this structure might not fully capture the delocalization of electrons in the molecule, potentially oversimplifying the bonding situation.
Implications of the Traditional Structure
The traditional Lewis structure implies a trigonal planar geometry for the SO3 molecule, with the sulfur atom at the center and the three oxygen atoms arranged around it. This geometry is consistent with the observed molecular shape of SO3, suggesting that this structure has some basis in reality. However, the fixed double bonds in this structure might not accurately represent the dynamic nature of electron distribution in the molecule.
Method 2: Resonance Structures with Delocalized Electrons
A more nuanced approach to the SO3 Lewis structure involves drawing resonance structures that reflect the delocalization of electrons across the molecule. This method acknowledges that the double bonds between sulfur and oxygen are not fixed but are instead distributed among all three oxygen atoms, resulting in a more realistic representation of the molecule’s electronic configuration.
By drawing multiple resonance structures and considering them as contributors to the overall hybrid structure, this approach better captures the molecular orbital picture of SO3. Each resonance structure satisfies the octet rule and minimizes formal charges, contributing to a more comprehensive understanding of the molecule's properties and reactivity.
Delocalization and Its Implications
The delocalization of electrons in the SO3 molecule, as represented by resonance structures, has significant implications for its reactivity and molecular properties. This delocalization leads to a stabilization of the molecule, making it less reactive than might be expected based on a single, fixed Lewis structure. Furthermore, the distribution of electrons across the molecule influences its polarity, with the sulfur atom bearing a partial positive charge and the oxygen atoms carrying partial negative charges.
Method 3: Bent’s Rule and the Importance of Hybridization
Bent’s rule offers another perspective on the SO3 Lewis structure, emphasizing the role of hybridization in determining the molecular geometry and electron distribution. According to Bent’s rule, the hybridization of the central atom (sulfur, in this case) is influenced by the electronegativities of the surrounding atoms (oxygen), leading to a situation where the s-character of the hybrid orbitals increases with the electronegativity of the bonded atoms.
This approach highlights the importance of considering the hybridization state of the sulfur atom and how it affects the distribution of electrons within the molecule. By understanding the hybridization and its implications for the molecular orbitals, one can gain deeper insights into the chemical properties of SO3, including its reactivity towards other molecules and its behavior in different chemical environments.
| Method | Description | Implications |
|---|---|---|
| Traditional | Double bonds between S and two O atoms | Trigonal planar geometry, potential oversimplification of electron distribution |
| Resonance Structures | Delocalization of electrons across the molecule | More realistic representation of electronic configuration, stabilization of the molecule |
| Bent's Rule | Hybridization and its effect on electron distribution | Insights into molecular geometry, reactivity, and chemical properties |
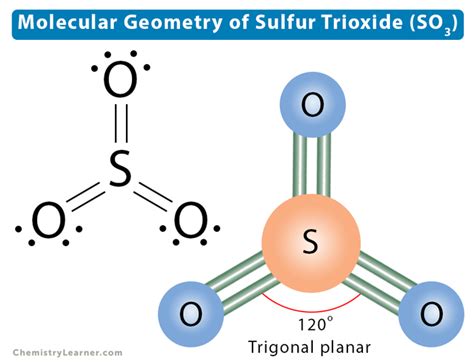
In conclusion, the SO3 Lewis structure can be represented in various ways, each with its strengths and limitations. By considering these different approaches and the principles underlying them, chemists can develop a more comprehensive understanding of the molecule's properties and behavior, facilitating predictions of its reactivity and interactions in different chemical contexts.
What is the significance of the SO3 Lewis structure in understanding its chemical properties?
+The SO3 Lewis structure is crucial for understanding its chemical properties because it provides insights into the distribution of electrons, the nature of bonding, and the molecular geometry, all of which influence its reactivity and interactions with other molecules.
How does the delocalization of electrons in the SO3 molecule affect its stability and reactivity?
+The delocalization of electrons in SO3 leads to a stabilization of the molecule, reducing its reactivity compared to what might be expected from a single, fixed Lewis structure. This stabilization is a result of the distribution of electrons across the molecule, which minimizes formal charges and maximizes bond order.
What role does Bent's rule play in understanding the SO3 Lewis structure and its implications for chemical properties?
+Bent's rule emphasizes the importance of hybridization in determining the molecular geometry and electron distribution. By considering the hybridization state of the sulfur atom and its effect on the molecular orbitals, one can gain insights into the chemical properties of SO3, including its reactivity and behavior in different chemical environments.
Meta Description: Explore the different ways to draw the SO3 Lewis structure, including traditional, resonance, and Bent’s rule approaches, to understand its chemical properties and reactivity.