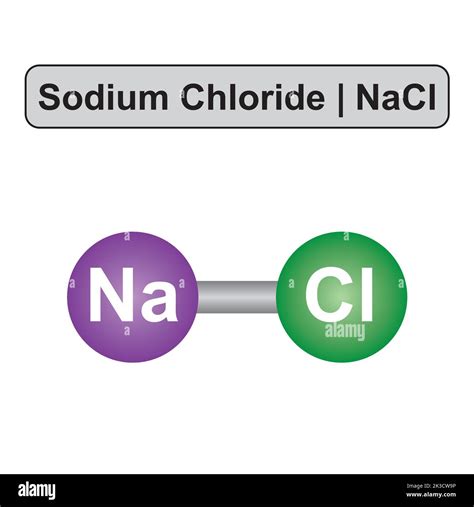
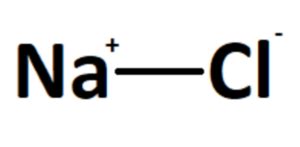Sodium chloride, commonly known as table salt, is a chemical compound with a wide range of applications in various industries, including food, pharmaceuticals, and manufacturing. The chemical formula for sodium chloride is NaCl, indicating that one sodium (Na) atom is bonded to one chlorine (Cl) atom. This compound is highly soluble in water and is essential for human health, as it helps regulate fluid balance and nerve function.
Naturally Occurring Sodium Chloride
Sodium chloride can be found naturally in the form of halite, also known as rock salt. It is often extracted from underground deposits through mining or solution mining, where water is injected into the deposit to dissolve the salt, and the resulting brine is pumped to the surface for processing. Sodium chloride is also a major component of seawater, with an average concentration of about 3.5% (35 grams per liter).
Sodium Chloride Chemical Structure
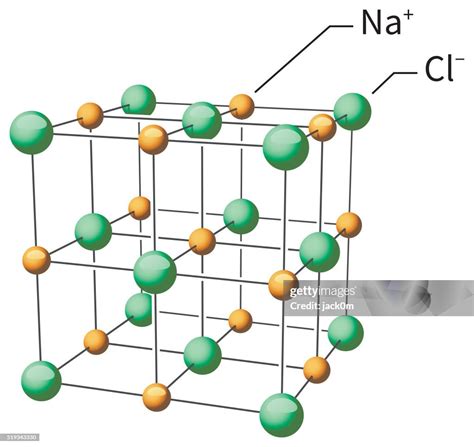
The chemical structure of sodium chloride consists of a lattice arrangement of sodium and chlorine ions. Each sodium ion is surrounded by six chlorine ions, and each chlorine ion is surrounded by six sodium ions. This ionic bonding between sodium and chlorine gives sodium chloride its high melting and boiling points, as well as its high solubility in water.
| Property | Value |
|---|---|
| Molecular Formula | NaCl |
| Molecular Weight | 58.44 g/mol |
| Melting Point | 800.7°C (1473.3 K) |
| Boiling Point | 1413°C (1686 K) at 101.3 kPa |
Key Points
- The chemical formula for sodium chloride is NaCl, indicating a 1:1 ratio of sodium to chlorine atoms.
- Sodium chloride is highly soluble in water, with a solubility of about 359 grams per liter at 20°C.
- The compound has a melting point of 800.7°C and a boiling point of 1413°C at standard pressure.
- Sodium chloride is essential for human health, playing a crucial role in regulating fluid balance and nerve function.
- The compound has a wide range of industrial applications, including the manufacture of plastics, textiles, and paper products.
Industrial Applications of Sodium Chloride
In addition to its use as a food seasoning and preservative, sodium chloride is used in a variety of industrial processes. It is a key component in the manufacture of plastics, such as polyvinyl chloride (PVC), and is used in the production of textiles, such as nylon and polyester. Sodium chloride is also used in the paper and pulp industry, where it helps to bleach and delignify wood pulp.
Pharmaceutical Applications of Sodium Chloride
Sodium chloride is also used in the pharmaceutical industry, where it is used as an excipient in the production of tablets and capsules. It is also used as a component in intravenous solutions, where it helps to maintain fluid balance and electrolyte levels in the body.
| Application | Description |
|---|---|
| Food Industry | Seasoning and preservative |
| Pharmaceutical Industry | Excipient and component of intravenous solutions |
| Textile Industry | Production of nylon and polyester |
| Paper and Pulp Industry | Bleaching and delignification of wood pulp |
In conclusion, sodium chloride is a highly versatile compound with a wide range of applications in various industries. Its unique chemical properties make it an essential component in many industrial processes, and its use is expected to continue to grow in the coming years.
What is the chemical formula for sodium chloride?
+The chemical formula for sodium chloride is NaCl, indicating a 1:1 ratio of sodium to chlorine atoms.
What are the main applications of sodium chloride?
+Sodium chloride has a wide range of applications, including the food industry, pharmaceutical industry, textile industry, and paper and pulp industry.
What is the solubility of sodium chloride in water?
+Sodium chloride is highly soluble in water, with a solubility of about 359 grams per liter at 20°C.