Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals. Sodium is the sixth most abundant element on Earth and makes up about 2.6% of the Earth's crust. It is highly reactive and readily loses one electron to form a positive ion, known as a cation. This reactivity is the reason why sodium is never found in its elemental form in nature, but rather in compounds such as sodium chloride (NaCl), also known as common table salt.
Physical and Chemical Properties of Sodium

Sodium has several distinct physical and chemical properties that set it apart from other elements. Its atomic mass is approximately 22.99 u (unified atomic mass units), and it has a melting point of 97.82°C and a boiling point of 883°C. Sodium is highly electropositive, meaning it readily loses electrons to form a positive ion. This property makes it highly reactive, especially with water, which it reacts with violently to produce sodium hydroxide and hydrogen gas. The reaction is highly exothermic, releasing a significant amount of heat and potentially igniting the hydrogen gas produced.
Sodium in Biological Systems
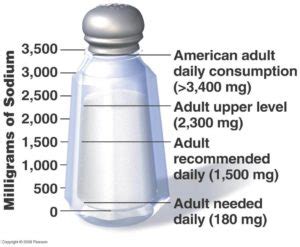
Sodium plays a critical role in biological systems, particularly in the human body. It is an essential nutrient and helps maintain fluid balance and blood pressure. Sodium ions also play a key role in nerve and muscle function, helping to transmit nerve impulses and contract muscles. The recommended daily intake of sodium varies by age, but for healthy individuals, the American Heart Association suggests consuming no more than 2,300 milligrams of sodium per day. Excessive sodium consumption can lead to hypertension (high blood pressure), heart disease, and stroke.
| Health Effects | Recommended Intake |
|---|---|
| Hypertension Risk | Less than 2,300 mg/day |
| Cardiovascular Health | Aim for 1,500 mg/day |
| Muscle and Nerve Function | Variable, based on individual needs |

Key Points
- Sodium is a highly reactive, soft, silvery-white metal that readily loses one electron to form a positive ion.
- It is essential for various bodily functions, including maintaining fluid balance, transmitting nerve impulses, and contracting muscles.
- The recommended daily intake of sodium is no more than 2,300 milligrams for healthy individuals, with a lower intake of 1,500 milligrams recommended for those at risk of hypertension.
- Sodium plays a critical role in maintaining proper blood pressure and can contribute to hypertension if consumed in excess.
- Regulation of sodium balance in the body is primarily managed by the kidneys, which adjust sodium excretion in the urine.
Industrial and Commercial Applications of Sodium
Beyond its biological importance, sodium has numerous industrial and commercial applications. It is used in the production of sodium carbonate (soda ash), sodium hydroxide (caustic soda), and sodium chloride (common salt), among other compounds. These chemicals are used in various industries, including manufacturing, water treatment, and food production. Sodium is also used in the manufacture of soap, paper, dyes, and textiles. Its high reactivity makes it useful in the production of other chemicals and in various chemical reactions.
Environmental Considerations
The extraction and use of sodium and its compounds can have environmental implications. The production of sodium hydroxide, for example, can lead to the release of chlorine gas, which is harmful to the environment. Similarly, the excessive use of sodium in agricultural fertilizers can lead to soil salinization, reducing the fertility of the land. Thus, it is crucial to manage sodium extraction and use in a way that minimizes environmental impact, including the implementation of pollution controls and sustainable agricultural practices.
| Application | Description |
|---|---|
| Manufacturing | Production of sodium compounds used in various industries. |
| Water Treatment | Sodium hydroxide is used to adjust the pH of water. |
| Food Production | Sodium chloride is used as a seasoning and preservative. |
In conclusion, sodium is a multifaceted element with critical roles in both biological systems and industrial applications. Its unique properties make it an essential component of various compounds used in manufacturing, agriculture, and consumer products. However, its high reactivity and potential environmental impact necessitate careful management and regulation of its use. As research continues to uncover the complexities of sodium's roles in human health and the environment, it is clear that this element will remain a vital and fascinating subject of study for years to come.
What are the primary health risks associated with excessive sodium consumption?
+Excessive sodium consumption is primarily linked to hypertension (high blood pressure), which can lead to heart disease and stroke. It can also affect kidney function and bone health.
How does the body regulate sodium levels?
+The body regulates sodium levels primarily through the kidneys, which adjust the amount of sodium excreted in the urine. Hormones such as aldosterone also play a role in sodium balance by promoting sodium retention in the body.
What are some common industrial applications of sodium?
+Sodium is used in the production of various chemicals, including sodium hydroxide and sodium carbonate. It is also used in the manufacture of soap, paper, and textiles, and in water treatment processes.