Solid dissolved in liquid examples are a fundamental concept in chemistry, particularly in the study of solutions. A solution is a homogeneous mixture of two or more substances, where one substance, the solute, is dissolved in another substance, the solvent. The solute can be a solid, liquid, or gas, while the solvent is typically a liquid. In this article, we will delve into the world of solid dissolved in liquid examples, exploring various scenarios and the principles that govern these interactions.
Introduction to Solid Dissolved in Liquid Examples
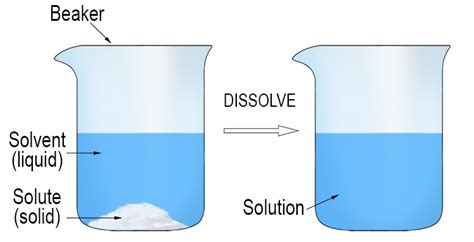
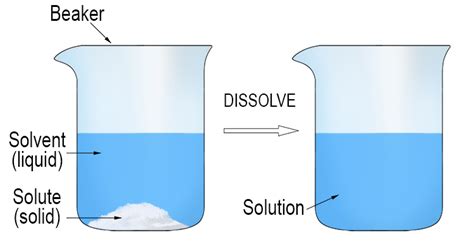
When a solid is dissolved in a liquid, the solid breaks down into its constituent particles, such as atoms, ions, or molecules, which then distribute evenly throughout the liquid. This process is known as dissolution. The resulting mixture is called a solution, which can exhibit unique properties that differ from those of the individual components. Solid dissolved in liquid examples are ubiquitous in our daily lives, ranging from sugar in water to medicinal compounds in pharmaceutical formulations.
Key Points
- Solid dissolved in liquid examples involve the dissolution of a solid solute in a liquid solvent.
- The solute breaks down into its constituent particles, which distribute evenly throughout the solvent.
- Solid dissolved in liquid examples are common in various industries, including food, pharmaceuticals, and manufacturing.
- Factors such as temperature, pressure, and solvent properties can influence the solubility of a solid in a liquid.
- Understanding solid dissolved in liquid examples is crucial for developing new products and processes in various fields.
Types of Solid Dissolved in Liquid Examples
There are several types of solid dissolved in liquid examples, classified based on the nature of the solute and solvent. For instance, a sugar-water solution is an example of a solid (sugar) dissolved in a liquid (water). Similarly, a saltwater solution is another example, where salt (sodium chloride) is dissolved in water. Other examples include medicinal compounds, such as acetaminophen, dissolved in water or other solvents to create pharmaceutical formulations.
| Example | Solute | Solvent |
|---|---|---|
| Sugar-water solution | Sugar (sucrose) | Water |
| Saltwater solution | Salt (sodium chloride) | Water |
| Medicinal formulation | Acetaminophen | Water or other solvents |

Factors Influencing Solid Dissolved in Liquid Examples
Several factors can influence the solubility of a solid in a liquid, including temperature, pressure, and solvent properties. For instance, increasing the temperature of a solution can increase the solubility of the solute, as the particles gain kinetic energy and move more freely. Conversely, decreasing the pressure can decrease the solubility of the solute, as the particles are less likely to interact with the solvent. The properties of the solvent, such as its polarity and molecular structure, can also affect the solubility of the solute.
Applications of Solid Dissolved in Liquid Examples
Solid dissolved in liquid examples have numerous applications in various industries, including food, pharmaceuticals, and manufacturing. For example, the food industry relies heavily on solid dissolved in liquid examples, such as sugar-water solutions, to create a wide range of products, from beverages to baked goods. In the pharmaceutical industry, medicinal compounds are often dissolved in solvents to create formulations that can be administered orally or topically. In manufacturing, solid dissolved in liquid examples are used to create a variety of products, such as cleaning solutions and coatings.
What is the difference between a solution and a suspension?
+A solution is a homogeneous mixture of two or more substances, where one substance is dissolved in another. A suspension, on the other hand, is a heterogeneous mixture, where the particles of one substance are dispersed throughout another substance, but not dissolved.
How does temperature affect the solubility of a solid in a liquid?
+Increasing the temperature of a solution can increase the solubility of the solute, as the particles gain kinetic energy and move more freely. Conversely, decreasing the temperature can decrease the solubility of the solute.
What are some common examples of solid dissolved in liquid examples in everyday life?
+Common examples of solid dissolved in liquid examples include sugar-water solutions, saltwater solutions, and medicinal formulations. These examples are ubiquitous in our daily lives, ranging from food and beverages to pharmaceuticals and cleaning products.
In conclusion, solid dissolved in liquid examples are a fundamental concept in chemistry, with numerous applications in various industries. Understanding the principles that govern these interactions is crucial for developing new products and processes. By recognizing the factors that influence the solubility of a solid in a liquid, we can create innovative solutions that improve our daily lives. As we continue to explore and discover new solid dissolved in liquid examples, we can unlock the full potential of these interactions and create a brighter future for generations to come.