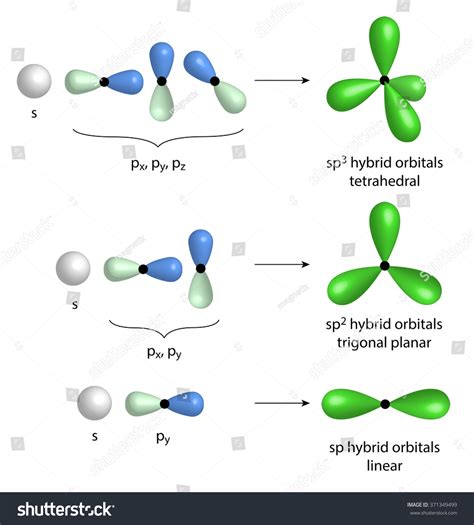
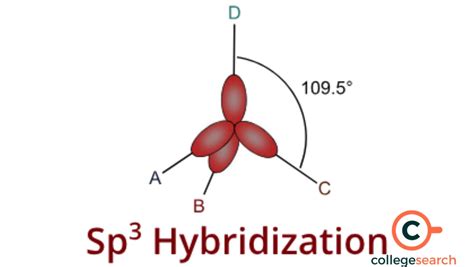
The concept of sp3 hybrid orbitals is a fundamental aspect of chemistry, particularly in understanding the bonding and structure of molecules. This phenomenon is crucial in explaining how atoms share electrons to form bonds, leading to the creation of various compounds. The term "sp3" refers to the mixing of one s orbital and three p orbitals, resulting in four equivalent hybrid orbitals. These hybrid orbitals are directed towards the corners of a tetrahedron, making them essential for the tetrahedral geometry seen in many molecules. In this article, we will delve into the workings of sp3 hybrid orbitals, exploring their significance, formation, and applications in chemistry.
Key Points
- Sp3 hybrid orbitals are formed by the combination of one s orbital and three p orbitals, leading to a tetrahedral arrangement.
- The process of hybridization allows for the creation of stronger bonds due to the increased overlap between orbitals.
- Sp3 hybridization is commonly observed in molecules such as methane (CH4) and ammonia (NH3), where it facilitates the tetrahedral and trigonal pyramidal geometries, respectively.
- The energy levels of sp3 hybrid orbitals are equivalent, enabling them to participate in bonding with similar effectiveness.
- Understanding sp3 hybridization is vital for predicting molecular shapes, reactivity, and physical properties, making it a cornerstone of organic and inorganic chemistry.
Formation of Sp3 Hybrid Orbitals
The formation of sp3 hybrid orbitals involves the mixing of atomic orbitals. In a carbon atom, for example, the 2s orbital and the three 2p orbitals (2px, 2py, 2pz) combine to form four sp3 hybrid orbitals. This process is energetically favorable because it allows the electrons to be distributed in a way that maximizes bonding and minimizes repulsion. The resulting sp3 hybrid orbitals are degenerate, meaning they have the same energy, and are oriented in space to maximize their separation, adopting a tetrahedral arrangement. This geometry is particularly stable and is a common motif in organic chemistry, where carbon atoms frequently exhibit sp3 hybridization.
Significance of Sp3 Hybrid Orbitals in Bonding
The significance of sp3 hybrid orbitals lies in their ability to form strong sigma bonds. The tetrahedral arrangement of these orbitals allows for the efficient overlap with orbitals from other atoms, leading to the formation of stable molecules. For instance, in methane (CH4), each of the four sp3 hybrid orbitals of the carbon atom overlaps with a hydrogen 1s orbital, resulting in four equivalent C-H bonds. This symmetry and the strength of these bonds contribute to the stability and the tetrahedral geometry of the methane molecule. Similarly, in ammonia (NH3), the nitrogen atom undergoes sp3 hybridization, but due to the presence of a lone pair, the molecule adopts a trigonal pyramidal geometry.
| Molecule | Geometry | Bond Angles |
|---|---|---|
| Methane (CH4) | Tetrahedral | 109.5° |
| Ammonia (NH3) | Trigonal Pyramidal | 107.3° |
Applications of Sp3 Hybrid Orbitals
The applications of sp3 hybrid orbitals are vast and varied, underpinning much of organic and inorganic chemistry. In organic chemistry, the understanding of sp3 hybridization is crucial for predicting the structures and reactivities of molecules. For example, the stability of alkanes (saturated hydrocarbons) can be attributed to the sp3 hybridization of carbon atoms, which leads to strong and stable C-C and C-H bonds. Furthermore, the concept of sp3 hybridization is essential in understanding the mechanisms of various organic reactions, including substitution and elimination reactions, where the hybridization state of the carbon atom involved in the reaction can significantly influence the reaction pathway and outcome.
Conclusion and Future Directions
In conclusion, sp3 hybrid orbitals play a pivotal role in chemistry, facilitating the understanding of molecular structures, bonding, and reactivity. The tetrahedral geometry associated with sp3 hybridization is a fundamental concept that underlies the properties of many molecules. As chemistry continues to evolve, the understanding of sp3 hybrid orbitals will remain essential for the development of new materials, drugs, and technologies. Future research directions may include exploring how sp3 hybridization influences the properties of materials at the nanoscale and how it can be manipulated to create molecules with specific functionalities.
What is the primary advantage of sp3 hybridization in terms of molecular stability?
+The primary advantage of sp3 hybridization is the formation of strong sigma bonds due to the efficient overlap of sp3 hybrid orbitals, leading to increased molecular stability.
Can sp3 hybridization occur in atoms other than carbon?
+Yes, sp3 hybridization can occur in other atoms, such as nitrogen in ammonia (NH3) and oxygen in water (H2O), although the resulting geometries may differ due to the presence of lone pairs.
How does sp3 hybridization influence the reactivity of a molecule?
+Sp3 hybridization can influence the reactivity of a molecule by affecting the availability of electrons for bonding and the stability of intermediates formed during reactions. The specific effect depends on the molecule and the reaction involved.