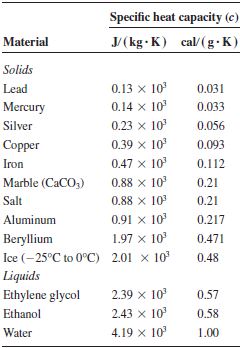
The specific heat value of copper, denoted as Cu, is a critical parameter in understanding its thermal properties. Copper, with its atomic number 29 and atomic mass 63.546 u, is a widely used metal in various industries due to its high electrical conductivity, corrosion resistance, and thermal conductivity. The specific heat capacity of copper is defined as the amount of heat per unit mass required to raise its temperature by one degree Celsius. This property is essential in designing and optimizing systems where copper is used as a material, such as in heat exchangers, electrical wiring, and electronic components.
Specific Heat Capacity of Copper
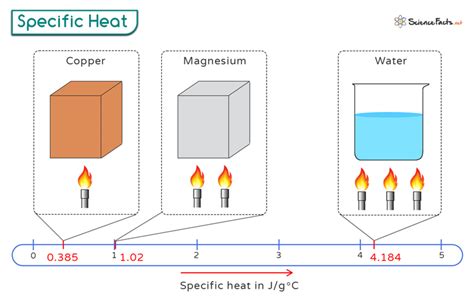
The specific heat capacity of copper at room temperature (20°C or 293 K) is approximately 0.385 J/g·K. This value indicates that 0.385 joules of heat energy are required to increase the temperature of one gram of copper by one Kelvin (or one degree Celsius). It’s worth noting that the specific heat capacity of copper, like many metals, can vary slightly with temperature. However, for most practical applications, the value at room temperature is sufficiently accurate for calculations and designs.
Variation with Temperature
While the specific heat capacity of copper at room temperature is well-documented, its value does change with temperature, albeit slightly. At higher temperatures, the specific heat capacity of copper increases. For instance, at 100°C (373 K), the specific heat capacity of copper is about 0.395 J/g·K, showing a slight increase from its value at room temperature. This variation is due to the increased vibrational motion of the atoms within the copper lattice as temperature increases, requiring more energy to achieve the same temperature rise.
| Temperature (°C) | Specific Heat Capacity (J/g·K) |
|---|---|
| 20 | 0.385 |
| 100 | 0.395 |
| 200 | 0.410 |
Key Points
- The specific heat capacity of copper at room temperature is approximately 0.385 J/g·K.
- This value increases slightly with temperature, reaching about 0.395 J/g·K at 100°C.
- Understanding the specific heat capacity of copper is crucial for thermal management in various applications.
- The variation of specific heat capacity with temperature must be considered in high-temperature applications.
- Accurate values of specific heat capacity are essential for designing efficient systems that utilize copper.
In conclusion, the specific heat value of copper is a vital parameter that influences its performance in a wide range of applications. By understanding how this property varies with temperature, engineers and designers can create more efficient and reliable systems that leverage the unique thermal and electrical properties of copper.
What is the significance of specific heat capacity in materials science?
+The specific heat capacity is crucial as it determines how much heat energy is required to change the temperature of a material, influencing its thermal management and efficiency in various applications.
How does the specific heat capacity of copper compare to other metals?
+Copper has a relatively high specific heat capacity compared to some metals but lower than others. For example, aluminum has a higher specific heat capacity than copper, while steel has a lower one, making each material suitable for different applications based on their thermal properties.
What are some common applications where the specific heat capacity of copper is particularly important?
+Copper’s specific heat capacity plays a critical role in applications such as heat exchangers, electronic components, and electrical wiring, where efficient heat transfer and thermal management are essential for performance and longevity.