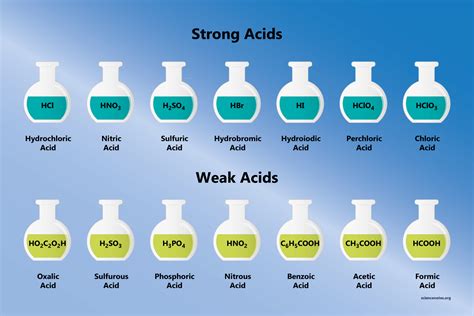
The reaction between a strong acid and a strong base is a fundamental concept in chemistry, particularly in the field of acid-base reactions. This type of reaction is characterized by the complete dissociation of both the acid and the base in water, resulting in the formation of a salt and water. The strong acid-strong base reaction is a neutralization reaction, where the hydrogen ions (H+) from the acid combine with the hydroxide ions (OH-) from the base to form water (H2O).
In a strong acid-strong base reaction, the acid is typically a mineral acid such as hydrochloric acid (HCl), sulfuric acid (H2SO4), or nitric acid (HNO3), while the base is usually a metal hydroxide like sodium hydroxide (NaOH) or potassium hydroxide (KOH). When these two substances are mixed, they react vigorously to form a salt and water. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) can be represented by the following equation: HCl + NaOH → NaCl + H2O. This reaction is highly exothermic, releasing heat and often resulting in a temperature increase in the surrounding environment.
Key Points
- The reaction between a strong acid and a strong base is a neutralization reaction, resulting in the formation of a salt and water.
- Strong acids and strong bases completely dissociate in water, meaning they fully ionize into their respective ions.
- The reaction is highly exothermic, releasing heat and often causing a temperature increase in the surroundings.
- Examples of strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3), while examples of strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
- The reaction is typically represented by the equation: H+ (from the acid) + OH- (from the base) → H2O (water).
Chemical Principles Behind Strong Acid-Strong Base Reactions
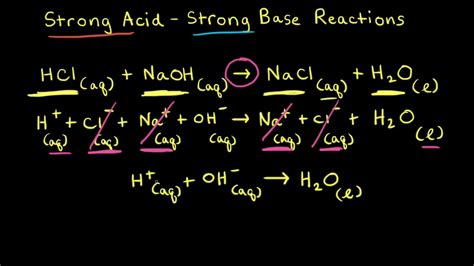
The chemical principles behind strong acid-strong base reactions are rooted in the Arrhenius definition of acids and bases. According to this definition, an acid is a substance that increases the concentration of hydrogen ions (H+) in a solution, while a base is a substance that increases the concentration of hydroxide ions (OH-). Strong acids and strong bases are characterized by their ability to completely dissociate in water, meaning they fully ionize into their respective ions. This complete dissociation is what drives the reaction between a strong acid and a strong base, as the hydrogen ions from the acid and the hydroxide ions from the base readily combine to form water.
Equilibrium and Stoichiometry in Strong Acid-Strong Base Reactions
In the context of strong acid-strong base reactions, equilibrium and stoichiometry play crucial roles. Since both the acid and the base are strong, they completely dissociate, and the reaction goes to completion. This means that the equilibrium constant (K) for the reaction is very large, indicating that the reaction strongly favors the products. The stoichiometry of the reaction is also straightforward, with a 1:1 mole ratio between the acid and the base. This simplicity in stoichiometry makes it easier to predict the outcome of the reaction and to calculate the amounts of reactants and products involved.
| Reactant | Product |
|---|---|
| Hydrochloric Acid (HCl) | Sodium Chloride (NaCl) |
| Sodium Hydroxide (NaOH) | Water (H2O) |
Practical Applications of Strong Acid-Strong Base Reactions

Strong acid-strong base reactions have numerous practical applications across various industries. In the manufacturing sector, these reactions are used in the production of salts, which are essential components in many products, including detergents, pharmaceuticals, and food additives. Additionally, the neutralization of acidic or basic wastewater is a critical application, as it helps in maintaining environmental safety and preventing pollution. The understanding of strong acid-strong base reactions is also fundamental in laboratory settings, where it is used in titrations—a method for determining the concentration of a substance by reacting it with a known amount of another substance until the reaction is complete.
Environmental and Industrial Considerations
From an environmental perspective, the ability to neutralize acidic or basic substances is crucial for maintaining the pH balance of ecosystems. Strong acid-strong base reactions are used in wastewater treatment to adjust the pH of effluents before they are discharged into water bodies, thereby protecting aquatic life. In industrial processes, controlling the pH through neutralization reactions is essential for optimizing reaction conditions, ensuring product quality, and preventing equipment corrosion.
What are the key characteristics of a strong acid-strong base reaction?
+A strong acid-strong base reaction is characterized by the complete dissociation of both the acid and the base in water, resulting in the formation of a salt and water. It is a neutralization reaction that is highly exothermic.
What are some examples of strong acids and strong bases?
+Examples of strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3), while examples of strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
What is the significance of stoichiometry in strong acid-strong base reactions?
+The stoichiometry of strong acid-strong base reactions is straightforward, with a 1:1 mole ratio between the acid and the base, making it easier to predict the outcome of the reaction and to calculate the amounts of reactants and products involved.
In conclusion, strong acid-strong base reactions are a fundamental aspect of chemistry, underpinning numerous industrial, environmental, and laboratory applications. Understanding the principles behind these reactions, including their stoichiometry, equilibrium, and practical applications, is essential for chemists and professionals in related fields. By recognizing the importance of these reactions and their role in maintaining environmental balance and driving industrial processes, we can better appreciate the intricate chemistry that surrounds us.