The concept of temperature is deeply rooted in the kinetic energy of particles within a substance. As we delve into the world of thermodynamics, it becomes apparent that temperature is not just a measure of how hot or cold something feels, but rather a quantifiable property that reflects the average kinetic energy of the particles that make up a substance. In this article, we will explore the intricate relationship between temperature and kinetic energy, and how this understanding has far-reaching implications in various fields of science and engineering.
Introduction to Kinetic Energy and Temperature

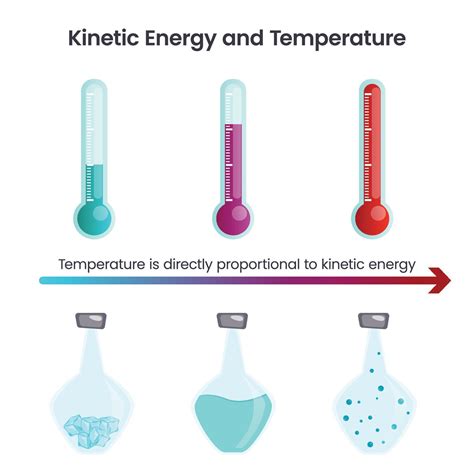
The kinetic energy of a particle is a measure of its energy due to motion. In a substance, the particles are in constant motion, colliding with each other and transferring energy. The temperature of a substance is a direct measure of the average kinetic energy of its particles. The higher the temperature, the greater the average kinetic energy of the particles, and vice versa. This fundamental relationship is described by the kinetic theory of gases, which states that the temperature of a gas is directly proportional to the average kinetic energy of its molecules.
Kinetic Theory of Gases and Temperature
The kinetic theory of gases provides a detailed understanding of the behavior of gases and how they respond to changes in temperature. According to this theory, the temperature of a gas is a measure of the average kinetic energy of its molecules. The kinetic energy of a molecule is given by the equation: KE = (1⁄2)mv^2, where m is the mass of the molecule and v is its velocity. The average kinetic energy of the molecules in a gas is directly proportional to the temperature of the gas, and is given by the equation: KE_avg = (3⁄2)kT, where k is the Boltzmann constant and T is the temperature in Kelvin.
| Physical Quantity | Symbol | Unit |
|---|---|---|
| Kinetic Energy | KE | Joules (J) |
| Mass | m | Kilograms (kg) |
| Velocity | v | Meters per second (m/s) |
| Boltzmann Constant | k | 1.380649 × 10^(-23) J/K |
| Temperature | T | Kelvin (K) |
Key Points
- The temperature of a substance is a direct measure of the average kinetic energy of its particles.
- The kinetic energy of a particle is a measure of its energy due to motion.
- The kinetic theory of gases provides a detailed understanding of the behavior of gases and how they respond to changes in temperature.
- The average kinetic energy of the molecules in a gas is directly proportional to the temperature of the gas.
- Understanding the relationship between temperature and kinetic energy has far-reaching implications in various fields of science and engineering.
Applications of Temperature and Kinetic Energy

The relationship between temperature and kinetic energy has numerous applications in various fields of science and engineering. In chemistry, the temperature of a reaction can affect the rate of reaction and the yield of products. In materials science, the temperature of a material can affect its mechanical properties, such as strength and ductility. In engineering, the temperature of a system can affect its efficiency and performance.
Chemical Reactions and Temperature
In chemical reactions, the temperature can affect the rate of reaction and the yield of products. Generally, an increase in temperature will increase the rate of reaction, as the particles have more kinetic energy and are more likely to collide and react. However, if the temperature is too high, the reaction can become less efficient, as the particles may have too much kinetic energy and collide too frequently, leading to the formation of unwanted byproducts.
For example, the Haber-Bosch process, which is used to produce ammonia, requires a temperature of around 450°C to achieve optimal yields. At this temperature, the nitrogen and hydrogen molecules have sufficient kinetic energy to react and form ammonia, but not so much that they form unwanted byproducts.
What is the relationship between temperature and kinetic energy?
+The temperature of a substance is a direct measure of the average kinetic energy of its particles. The higher the temperature, the greater the average kinetic energy of the particles, and vice versa.
How does temperature affect chemical reactions?
+Temperature can affect the rate of reaction and the yield of products in chemical reactions. Generally, an increase in temperature will increase the rate of reaction, as the particles have more kinetic energy and are more likely to collide and react.
What are some applications of the relationship between temperature and kinetic energy?
+The relationship between temperature and kinetic energy has numerous applications in various fields of science and engineering, including chemistry, materials science, and engineering.
Meta description suggestion: “Discover the relationship between temperature and kinetic energy, and how it affects chemical reactions and materials properties.” (140-155 characters)