The Belmont Report, published in 1979, is a seminal document in the history of medical ethics, outlining the fundamental principles and guidelines for the protection of human subjects in biomedical and behavioral research. The report was created by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, established by the United States government in response to the revelation of the Tuskegee syphilis study and other ethical atrocities. The Belmont Report's principles have had a profound impact on the conduct of research involving human subjects, shaping the ethical landscape of medical and scientific inquiry.
Background and Context

The Tuskegee syphilis study, which began in the 1930s and continued until its exposure in 1972, involved the withholding of treatment from African American men with syphilis, without their knowledge or consent. This egregious breach of ethical standards led to widespread outrage and calls for reform. In response, the National Research Act of 1974 was enacted, establishing the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The commission’s mandate was to identify the basic ethical principles that should guide the conduct of research involving human subjects and to develop guidelines for the implementation of these principles.
Core Principles
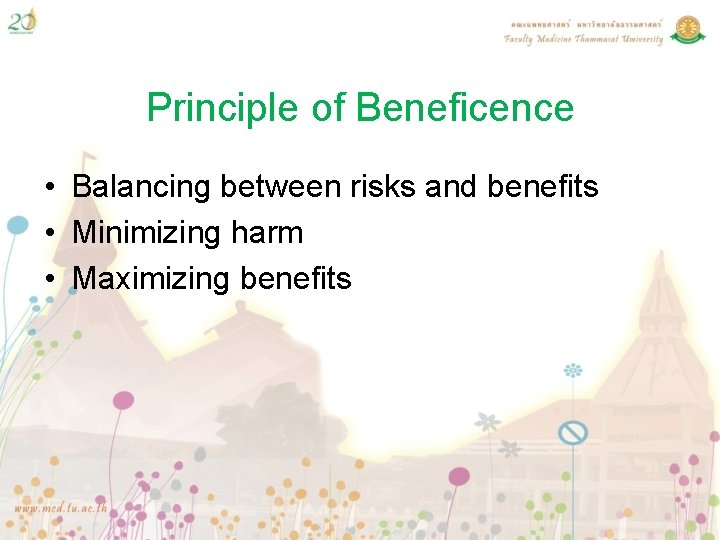
The Belmont Report identifies three core principles as the foundation for the ethical conduct of research involving human subjects: respect for persons, beneficence, and justice. Respect for persons recognizes the autonomy and dignity of individuals, requiring that they be treated as ends in themselves, rather than means to an end. This principle demands that researchers obtain informed consent from participants, ensuring that they understand the risks and benefits of the research and can make an informed decision about their participation. Beneficence obliges researchers to promote the well-being and safety of participants, minimizing harm and maximizing benefits. The principle of justice requires that the benefits and burdens of research be distributed fairly, with no group or individual shouldering a disproportionate share of the risks or reaping an unfair share of the benefits.
| Principle | Description |
|---|---|
| Respect for Persons | Recognizes autonomy and dignity, requiring informed consent |
| Beneficence | Promotes well-being and safety, minimizing harm and maximizing benefits |
| Justice | Distributes benefits and burdens fairly, ensuring no group bears disproportionate risk |

Application and Impact
The Belmont Report’s principles have had a profound impact on the conduct of research involving human subjects. The report’s emphasis on informed consent, for example, has led to the development of rigorous protocols for obtaining consent, ensuring that participants are fully aware of the risks and benefits of the research. The principle of beneficence has guided the development of safety protocols and procedures for minimizing harm to participants. The principle of justice has informed the design of research studies, ensuring that the benefits and burdens are distributed fairly and that no group is unfairly burdened or benefited.
Challenges and Limitations
Despite the significant advances in protecting human subjects, challenges and limitations remain. The process of obtaining informed consent, for example, can be complex and time-consuming, particularly in cases where participants may not fully understand the risks and benefits of the research. Additionally, the principle of justice can be difficult to apply in practice, particularly in cases where the benefits and burdens of research are not easily quantifiable. Furthermore, the increasing globalization of research has raised new challenges, such as ensuring that research is conducted in accordance with local laws and regulations, while also respecting the cultural and social norms of diverse populations.
Key Points
- The Belmont Report's principles of respect for persons, beneficence, and justice provide a foundation for the ethical conduct of research involving human subjects.
- Informed consent is a critical component of research, ensuring that participants understand the risks and benefits of the research.
- The principle of beneficence guides the development of safety protocols and procedures for minimizing harm to participants.
- The principle of justice informs the design of research studies, ensuring that the benefits and burdens are distributed fairly.
- Challenges and limitations remain, including the complexity of obtaining informed consent and the difficulty of applying the principle of justice in practice.
Conclusion and Future Directions
The Belmont Report’s principles have had a lasting impact on the conduct of research involving human subjects, shaping the ethical landscape of medical and scientific inquiry. As research continues to evolve and become increasingly global, it is essential that these principles are adapted and applied in new and innovative ways. By continuing to prioritize the protection of human subjects and upholding the principles of respect for persons, beneficence, and justice, researchers can ensure that their work is conducted with the highest ethical standards, promoting the well-being and safety of participants and advancing our understanding of the world around us.
What are the core principles of the Belmont Report?
+The Belmont Report identifies three core principles: respect for persons, beneficence, and justice.
Why is informed consent important in research?
+Informed consent ensures that participants understand the risks and benefits of the research and can make an informed decision about their participation.
How do the principles of the Belmont Report apply to global research?
+The principles of the Belmont Report must be adapted and applied in new and innovative ways to ensure that research is conducted with the highest ethical standards, respecting the cultural and social norms of diverse populations.