The thermal conductivity of water is a fundamental property that plays a crucial role in various natural and industrial processes. It is a measure of the ability of water to conduct heat, which is essential for understanding and predicting the behavior of water in different environments. In this article, we will delve into the concept of thermal conductivity, its significance, and the factors that influence the thermal conductivity of water.
Thermal conductivity is defined as the ability of a material to conduct heat, which is measured in units of watts per meter per degree Celsius (W/m°C). The thermal conductivity of water is relatively high compared to other liquids, which makes it an excellent heat transfer medium. This property is essential for various applications, including cooling systems, heat exchangers, and thermal energy storage. The thermal conductivity of water is also critical in understanding and predicting the behavior of water in natural systems, such as oceans, lakes, and rivers.
Key Points
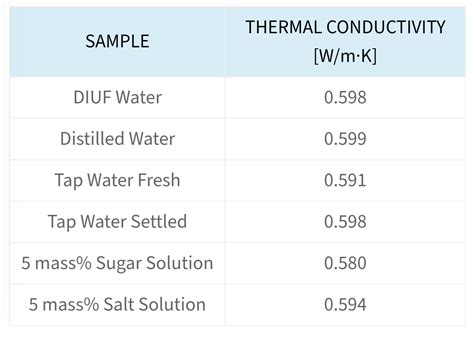
- The thermal conductivity of water is approximately 0.6 W/m°C at 20°C.
- Temperature is the primary factor that influences the thermal conductivity of water.
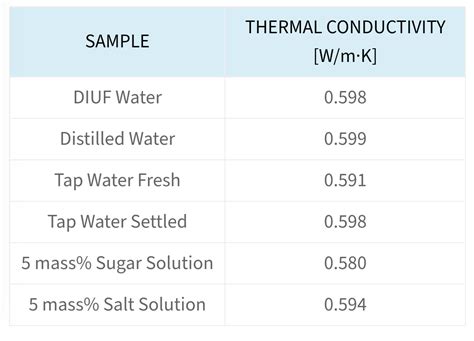
- The thermal conductivity of water decreases with increasing salinity.
- The thermal conductivity of water is affected by the presence of impurities and dissolved gases.
- Understanding the thermal conductivity of water is essential for various industrial and natural applications.
Factors Influencing Thermal Conductivity of Water
The thermal conductivity of water is influenced by several factors, including temperature, salinity, and the presence of impurities and dissolved gases. Temperature is the primary factor that affects the thermal conductivity of water, with a significant increase in thermal conductivity observed at higher temperatures. The thermal conductivity of water increases by approximately 2% per degree Celsius, which means that the thermal conductivity of water at 30°C is about 10% higher than at 20°C.
The presence of impurities and dissolved gases in water also affects its thermal conductivity. For example, the thermal conductivity of seawater is lower than that of pure water due to the presence of salts and other impurities. The thermal conductivity of water can also be affected by the presence of dissolved gases, such as oxygen and carbon dioxide, which can alter the thermal conductivity of water by changing its density and viscosity.
Temperature Dependence of Thermal Conductivity
The temperature dependence of thermal conductivity is a critical aspect of understanding the behavior of water in different environments. The thermal conductivity of water increases with increasing temperature, which means that water becomes a more effective heat transfer medium at higher temperatures. This property is essential for various industrial applications, including cooling systems and heat exchangers.
| Temperature (°C) | Thermal Conductivity (W/m°C) |
|---|---|
| 20 | 0.598 |
| 30 | 0.628 |
| 40 | 0.659 |
| 50 | 0.690 |

Applications of Thermal Conductivity of Water
The thermal conductivity of water has numerous applications in various industries, including power generation, chemical processing, and refrigeration. The high thermal conductivity of water makes it an excellent heat transfer medium, which is essential for efficient heat transfer in these applications. The thermal conductivity of water is also critical in understanding and predicting the behavior of water in natural systems, such as oceans, lakes, and rivers.
In addition to its industrial applications, the thermal conductivity of water is also essential for understanding and predicting the behavior of water in natural systems. For example, the thermal conductivity of water plays a critical role in the formation of ocean currents and the distribution of heat around the globe. The thermal conductivity of water is also essential for understanding and predicting the behavior of water in lakes and rivers, which is critical for managing water resources and predicting water quality.
Industrial Applications of Thermal Conductivity of Water
The thermal conductivity of water has numerous industrial applications, including cooling systems, heat exchangers, and thermal energy storage. The high thermal conductivity of water makes it an excellent heat transfer medium, which is essential for efficient heat transfer in these applications. The thermal conductivity of water is also critical in understanding and predicting the behavior of water in industrial processes, such as chemical processing and power generation.
In conclusion, the thermal conductivity of water is a fundamental property that plays a crucial role in various natural and industrial processes. Understanding the factors that influence the thermal conductivity of water is essential for optimizing the performance of cooling systems, heat exchangers, and thermal energy storage systems. The thermal conductivity of water is also critical in understanding and predicting the behavior of water in natural systems, such as oceans, lakes, and rivers.
What is the thermal conductivity of water at 20°C?
+The thermal conductivity of water at 20°C is approximately 0.598 W/m°C.
How does temperature affect the thermal conductivity of water?
+The thermal conductivity of water increases with increasing temperature, with a significant increase in thermal conductivity observed at higher temperatures.
What is the effect of salinity on the thermal conductivity of water?
+The thermal conductivity of water decreases with increasing salinity, which means that seawater has a lower thermal conductivity than pure water.
Meta description: “Discover the thermal conductivity of water and its significance in various industrial and natural processes. Learn about the factors that influence the thermal conductivity of water and its applications in cooling systems, heat exchangers, and thermal energy storage.” (148 characters)