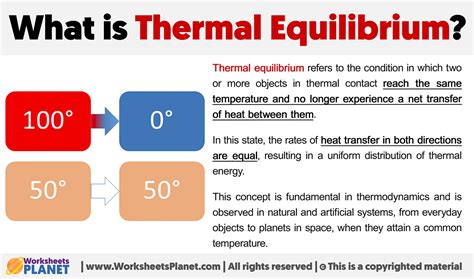
Thermal equilibrium is a fundamental concept in thermodynamics, describing a state where the temperature is uniform throughout a system or between systems in contact. This concept is crucial in understanding various natural phenomena and industrial processes, from the behavior of gases in the atmosphere to the efficiency of heat engines. At its core, thermal equilibrium is achieved when there is no net flow of heat between systems or within different parts of a system, indicating that the systems have reached the same temperature.
The definition of thermal equilibrium is grounded in the zeroth law of thermodynamics, which states that if two systems are in thermal equilibrium with a third system, then they are also in thermal equilibrium with each other. This law allows for the definition of a temperature scale, enabling the quantitative measurement of thermal equilibrium. The concept of thermal equilibrium underpins many thermodynamic processes and is essential for understanding how systems interact and reach a state of balance.
Key Points
- Thermal equilibrium occurs when the temperature is uniform throughout a system or between systems in contact.
- The zeroth law of thermodynamics provides the foundation for the concept of thermal equilibrium.
- Achieving thermal equilibrium implies there is no net heat flow between systems or parts of a system.
- Thermal equilibrium is crucial for understanding various natural and industrial processes.
- The concept allows for the definition of a temperature scale, facilitating quantitative measurements.
Understanding Thermal Equilibrium
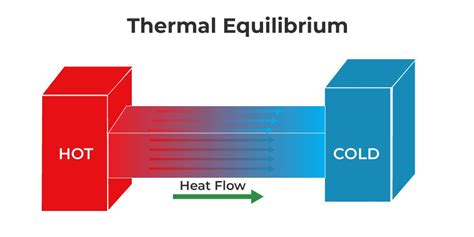
Thermal equilibrium is a dynamic state, meaning that while the system may appear to be at rest in terms of temperature, there are still molecular motions and interactions occurring. However, these interactions are balanced, resulting in no net change in the system’s temperature over time. The time it takes for a system to reach thermal equilibrium can vary significantly depending on the specific conditions, such as the material properties of the system and the environment in which it is placed.
The process of reaching thermal equilibrium involves heat transfer, which can occur through conduction, convection, or radiation. When two systems at different temperatures are brought into contact, heat flows from the system at the higher temperature to the system at the lower temperature until they reach the same temperature. This principle is fundamental to the design and operation of various thermal systems, including engines, refrigerators, and heat pumps.
Thermal Equilibrium and the Zeroth Law
The zeroth law of thermodynamics is crucial for defining and understanding thermal equilibrium. It essentially states that if system A is in thermal equilibrium with system C, and system B is also in thermal equilibrium with system C, then system A is in thermal equilibrium with system B. This principle allows for the comparison of temperatures between different systems and the establishment of a temperature scale, such as the Celsius or Kelvin scale, which are used universally to measure thermal equilibrium.
The implications of the zeroth law are far-reaching, enabling the development of thermometers and the measurement of temperature with precision. Temperature measurement is essential in science and engineering, as it allows for the prediction and control of thermal processes, ensuring efficiency, safety, and optimal performance in a wide range of applications.
| Concept | Description |
|---|---|
| Thermal Equilibrium | A state where the temperature is uniform throughout a system or between systems in contact. |
| Zeroth Law of Thermodynamics | If two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other. |
| Temperature Scale | A scale used to measure temperature, defined by the thermal equilibrium of systems. |
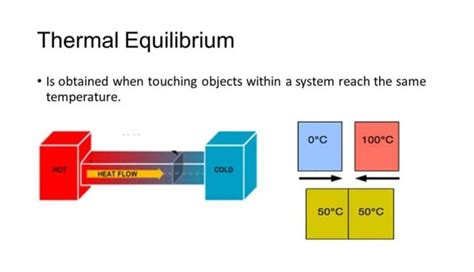
Applications of Thermal Equilibrium
Thermal equilibrium has numerous practical applications across various fields. In engineering, understanding thermal equilibrium is critical for the design of efficient thermal systems, such as heat exchangers, where the goal is to maximize heat transfer between two fluids while minimizing energy losses. In chemistry, thermal equilibrium is essential for understanding chemical reactions and the conditions under which they occur, as the equilibrium constant of a reaction is temperature-dependent.
In biology, thermal equilibrium plays a role in understanding how living organisms regulate their body temperature and respond to changes in their thermal environment. This is particularly important for mammals and birds, which are endotherms and maintain a relatively constant body temperature despite changes in the ambient temperature. The concept of thermal equilibrium also underlies the principles of climate science, where the balance between incoming solar radiation and outgoing infrared radiation determines the Earth's climate and weather patterns.
Challenges and Limitations
Despite its importance, achieving and maintaining thermal equilibrium can be challenging in certain situations. In systems where there are significant temperature gradients or where heat transfer is limited, achieving thermal equilibrium may take a long time or may not be possible at all. Additionally, in very small systems, such as those at the nanoscale, thermal fluctuations can become significant, making it difficult to achieve a stable thermal equilibrium state.
Furthermore, the concept of thermal equilibrium assumes that the system is in a state of internal equilibrium, where the temperature is uniform throughout. However, in real-world systems, there can be complexities such as non-uniform temperature distributions, phase transitions, or chemical reactions that complicate the achievement of thermal equilibrium. Understanding and addressing these challenges is crucial for the advancement of technologies that rely on thermal processes.
What is thermal equilibrium, and why is it important?
+Thermal equilibrium is a state where the temperature is uniform throughout a system or between systems in contact. It is important because it underlies our understanding of heat transfer, thermodynamic processes, and the behavior of systems at the molecular level, with applications in engineering, chemistry, biology, and climate science.
How does the zeroth law of thermodynamics relate to thermal equilibrium?
+The zeroth law of thermodynamics provides the foundation for the concept of thermal equilibrium by stating that if two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other. This principle allows for the definition of a temperature scale and the measurement of thermal equilibrium.
What are some practical applications of thermal equilibrium?
+Practical applications of thermal equilibrium include the design of efficient thermal systems in engineering, understanding chemical reactions and their conditions in chemistry, regulating body temperature in biology, and determining climate patterns in climate science.
In conclusion, thermal equilibrium is a fundamental concept in thermodynamics that underlies our understanding of heat transfer and the behavior of systems at various scales. Its applications are diverse, ranging from the design of thermal systems in engineering to the regulation of body temperature in living organisms. Understanding thermal equilibrium and its implications is crucial for advancing technologies and addressing challenges in fields that rely on thermal processes.