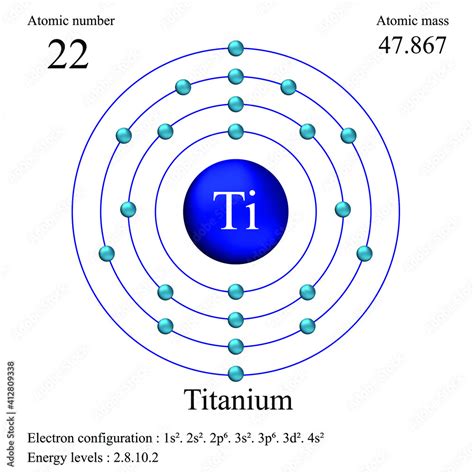
The electron configuration of titanium, a transition metal with the atomic number 22, is a crucial aspect of understanding its chemical properties and behavior. The electron configuration can be expressed in various ways, each providing insight into the arrangement of electrons in the atom. In this article, we will explore five different ways to express the electron configuration of titanium, delving into the nuances of each representation and their implications for titanium's chemical characteristics.
Introduction to Electron Configuration
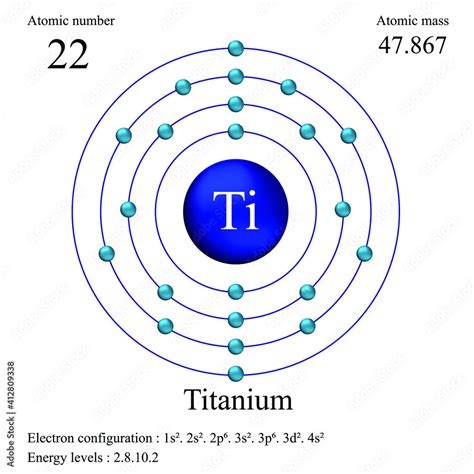
Electron configuration is the arrangement of electrons in an atom, which determines its chemical properties. It is typically expressed in a shorthand notation that indicates the energy level and orbital type of each electron. For titanium, the electron configuration is a reflection of its position in the periodic table, bridging the gap between the alkali metals and the transition metals.
Key Points
- The electron configuration of titanium is [Ar] 3d² 4s², reflecting its position in the periodic table.
- The Aufbau principle and the Pauli exclusion principle are fundamental in determining the electron configuration of atoms, including titanium.
- Hund's rule of maximum multiplicity plays a crucial role in predicting the electron configuration of atoms with unpaired electrons.
- Understanding the electron configuration of titanium is essential for predicting its chemical properties and reactivity.
- The electron configuration influences the formation of compounds and the oxidation states of titanium.
1. The Aufbau Principle and Electron Configuration
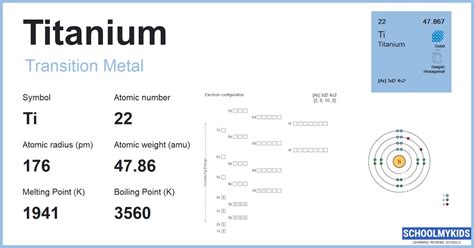
The Aufbau principle states that electrons fill the lowest available energy levels. Applying this principle to titanium, we start with the noble gas core [Ar], which represents the electrons in the first three energy levels (1s² 2s² 2p⁶ 3s² 3p⁶). The remaining two electrons in titanium occupy the 3d and 4s orbitals. According to the Aufbau principle, these electrons would occupy the 3d orbital first, due to its lower energy level, resulting in a configuration of [Ar] 3d² 4s². However, due to the specifics of atomic interactions, the actual configuration might slightly differ, emphasizing the importance of experimental verification.
Aufbau Principle Application
The application of the Aufbau principle provides a foundational understanding of electron configuration. It is a crucial tool in chemistry, allowing us to predict the electron configuration of atoms based on their atomic number. For titanium, the Aufbau principle helps in understanding why it exhibits certain chemical properties, such as its ability to form compounds with various oxidation states.
2. Hund’s Rule and Electron Configuration
Hund’s rule of maximum multiplicity states that when filling orbitals of equal energy, electrons occupy each orbital singly before pairing up, and they do so with parallel spins. Applying this rule to the electron configuration of titanium, we see that the two 3d electrons would occupy separate 3d orbitals with parallel spins, maximizing the multiplicity of the atom. This principle is essential for understanding the magnetic properties of titanium and its compounds.
Implications of Hund’s Rule
The implications of Hund’s rule on the electron configuration of titanium are significant. It helps in understanding the magnetic behavior of titanium, which is crucial for its applications in various fields, including materials science and physics. By maximizing the multiplicity, Hund’s rule influences the stability and reactivity of titanium compounds, making it a vital consideration in chemical reactions and compound formation.
3. The Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This principle is fundamental in determining the electron configuration of atoms, including titanium. It ensures that each electron in the 3d and 4s orbitals has a unique set of quantum numbers, which is crucial for the stability and chemical properties of titanium.
Pauli Exclusion Principle Application
The application of the Pauli exclusion principle to titanium’s electron configuration highlights its importance in chemistry. By ensuring that no two electrons have the same quantum numbers, the Pauli exclusion principle underpins the periodic table’s structure and the unique properties of each element, including titanium. This principle is essential for understanding why certain compounds are stable and others are not, guiding chemical synthesis and materials design.
4. Experimental Verification of Electron Configuration
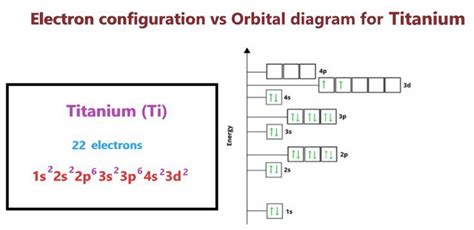
While theoretical principles provide a basis for predicting electron configurations, experimental verification is crucial. Spectroscopic methods, such as X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS), can directly observe the electron configuration of titanium. These experiments have confirmed the electron configuration of titanium as [Ar] 3d² 4s², validating the theoretical predictions and providing a deeper understanding of titanium’s chemical properties.
Experimental Methods
Experimental methods like XPS and UPS are vital tools in chemistry, offering direct evidence of an atom’s electron configuration. For titanium, these methods have been instrumental in confirming its electron configuration, which is essential for understanding its reactivity and the formation of compounds. By combining theoretical predictions with experimental evidence, scientists can develop a comprehensive understanding of titanium’s properties and behaviors.
5. Applications of Titanium’s Electron Configuration
The electron configuration of titanium has significant implications for its applications. Titanium’s ability to form compounds with various oxidation states, such as TiO₂ (titanium dioxide) and TiCl₄ (titanium tetrachloride), is directly related to its electron configuration. Understanding the electron configuration is essential for predicting the chemical properties of titanium and its compounds, which are used in a wide range of applications, from aerospace and biomedical implants to catalysts and pigments.
| Compound | Oxidation State | Application |
|---|---|---|
| TiO₂ | +4 | Pigments, Catalysts |
| TiCl₄ | +4 | Catalyst in Polymer Production |
| TiAl₆V₄ | Mixed | Aerospace Alloys |

What is the electron configuration of titanium?
+The electron configuration of titanium is [Ar] 3d² 4s², which reflects its position in the periodic table and influences its chemical properties.
Why is the Aufbau principle important for electron configuration?
+The Aufbau principle is essential because it provides a systematic way to predict the electron configuration of atoms based on their atomic number, helping to understand their chemical properties and behaviors.
How does Hund's rule affect the electron configuration of titanium?
+Hund's rule influences the electron configuration by maximizing the multiplicity of the atom, which affects the magnetic properties and stability of titanium compounds, making it a crucial consideration in chemical reactions and applications.
Meta Description Suggestion: Discover the five ways titanium’s electron configuration influences its chemical properties and applications, from the Aufbau principle to experimental verification, and understand how these principles impact its use in various industries. (149 characters)