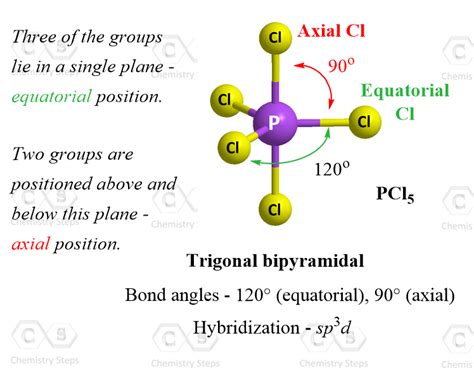
The trigonal bipyramidal bond angle is a fundamental concept in chemistry, specifically in the realm of molecular geometry. It describes the arrangement of five atoms or groups of atoms around a central atom, resulting in a specific three-dimensional shape. This concept is crucial in understanding the properties and behaviors of molecules, particularly in the fields of organic and inorganic chemistry. In this article, we will delve into the details of the trigonal bipyramidal bond angle, exploring its definition, significance, and implications in various chemical contexts.
Key Points
- The trigonal bipyramidal bond angle is approximately 120° for equatorial atoms and 90° for axial atoms.
- This bond angle is a result of the arrangement of five atoms or groups of atoms around a central atom.
- The trigonal bipyramidal shape is commonly observed in molecules with five ligands, such as phosphorus pentachloride (PCl5) and phosphorus pentafluoride (PF5).
- The bond angle is influenced by the repulsion between electron pairs, which is described by VSEPR theory.
- Understanding the trigonal bipyramidal bond angle is essential in predicting the physical and chemical properties of molecules.
Nature of the Trigonal Bipyramidal Bond Angle
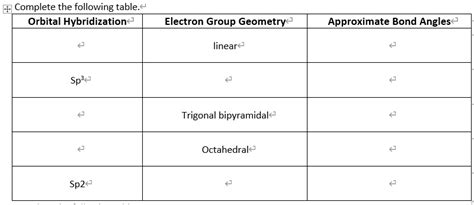
The trigonal bipyramidal bond angle is characterized by the arrangement of five atoms or groups of atoms around a central atom, typically a metal or nonmetal atom. This arrangement results in a three-dimensional shape with two distinct types of positions: axial and equatorial. The axial positions are located at the poles of the molecule, while the equatorial positions are situated around the middle of the molecule. The bond angle between the axial and equatorial atoms is approximately 90°, while the bond angle between the equatorial atoms is approximately 120°.
VSEPR Theory and Bond Angle
The Valence Shell Electron Pair Repulsion (VSEPR) theory provides a framework for understanding the trigonal bipyramidal bond angle. According to this theory, the repulsion between electron pairs around a central atom determines the molecular geometry. In the case of a trigonal bipyramidal molecule, the five electron pairs around the central atom arrange themselves to minimize repulsion, resulting in the characteristic bond angle. The VSEPR theory is a fundamental concept in chemistry, and its application to the trigonal bipyramidal bond angle is a testament to its utility in predicting molecular geometry.
| Atom/Ligand | Bond Angle |
|---|---|
| Axial | 90° |
| Equatorial | 120° |
| Axial-Equatorial | 90° |
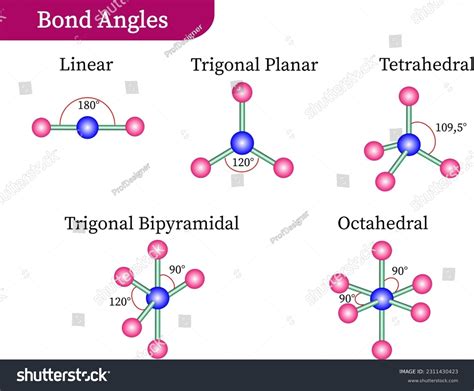
Implications of the Trigonal Bipyramidal Bond Angle
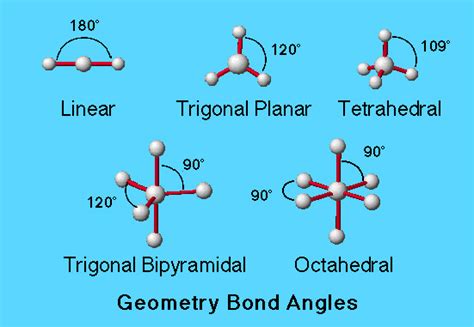
The trigonal bipyramidal bond angle has significant implications in various chemical contexts. For instance, the bond angle influences the reactivity of molecules, with axial atoms being more reactive than equatorial atoms. Additionally, the trigonal bipyramidal shape is commonly observed in molecules with five ligands, such as phosphorus pentachloride (PCl5) and phosphorus pentafluoride (PF5). These molecules exhibit unique properties, such as high reactivity and stability, which are influenced by the trigonal bipyramidal bond angle.
Phosphorus Pentachloride (PCl5) as an Example
Phosphorus pentachloride (PCl5) is a classic example of a molecule with a trigonal bipyramidal shape. The molecule consists of a central phosphorus atom surrounded by five chlorine atoms, with two axial and three equatorial positions. The bond angle between the axial and equatorial chlorine atoms is approximately 90°, while the bond angle between the equatorial chlorine atoms is approximately 120°. This arrangement results in a highly reactive molecule, which is commonly used as a chlorinating agent in organic synthesis.
What is the significance of the trigonal bipyramidal bond angle in chemistry?
+The trigonal bipyramidal bond angle is significant in chemistry because it influences the physical and chemical properties of molecules, such as their reactivity and stability. Understanding this concept is essential for chemists and researchers working in various fields, including organic synthesis, materials science, and pharmacology.
What is the VSEPR theory, and how does it relate to the trigonal bipyramidal bond angle?
+The VSEPR theory is a framework for understanding the arrangement of electron pairs around a central atom. According to this theory, the repulsion between electron pairs determines the molecular geometry. In the case of a trigonal bipyramidal molecule, the VSEPR theory predicts the characteristic bond angle, which is approximately 90° for axial atoms and 120° for equatorial atoms.
What are some examples of molecules with a trigonal bipyramidal shape?
+Phosphorus pentachloride (PCl5) and phosphorus pentafluoride (PF5) are classic examples of molecules with a trigonal bipyramidal shape. These molecules consist of a central phosphorus atom surrounded by five ligands, with two axial and three equatorial positions.
In conclusion, the trigonal bipyramidal bond angle is a fundamental concept in chemistry, with significant implications in various chemical contexts. Understanding this concept is essential for chemists and researchers working in fields such as organic synthesis, materials science, and pharmacology. The VSEPR theory provides a framework for predicting the molecular geometry of trigonal bipyramidal molecules, and the bond angle has a profound influence on the physical and chemical properties of these molecules.