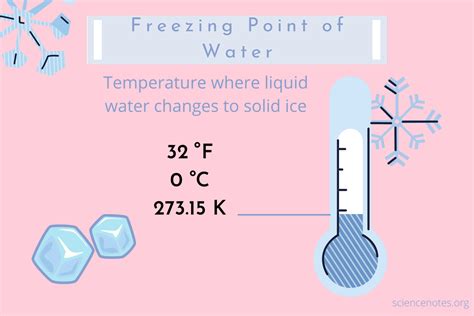
The water freezing point, a fundamental concept in physics and chemistry, has fascinated scientists and researchers for centuries. At its core, the freezing point of water is the temperature at which water changes state from a liquid to a solid, forming ice. This process is crucial in understanding various natural phenomena, from the formation of glaciers to the preservation of food. In this article, we will delve into the intricacies of the water freezing point, exploring its definition, factors influencing it, and its significance in different fields.
Key Points
- The freezing point of water is 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F) under standard atmospheric pressure.
- The freezing point can be influenced by factors such as pressure, dissolved substances, and the presence of ice nucleating agents.
- Understanding the freezing point of water is crucial in fields like chemistry, biology, and environmental science.
- The freezing point depression is a phenomenon where the freezing point of a solution is lower than that of the pure solvent.
- Research into the water freezing point has applications in cryopreservation, climate modeling, and the development of antifreeze materials.
Definition and Standard Conditions

The standard freezing point of water is defined as 0°C or 32°F at standard atmospheric pressure, which is approximately 101.325 kPa. This value serves as a reference point for various scientific and engineering applications. However, it’s essential to note that the freezing point can vary under different conditions. For instance, an increase in pressure can lower the freezing point, a phenomenon observed in the formation of ice at the bottom of deep lakes and oceans.
Factors Influencing the Freezing Point
Several factors can influence the freezing point of water, including dissolved substances, pressure, and the presence of ice nucleating agents. Dissolved substances, such as salts or sugars, can lower the freezing point of water through a process known as freezing point depression. This phenomenon is exploited in the production of antifreeze solutions for vehicles and in the preservation of food. Pressure also plays a significant role, as an increase in pressure can decrease the freezing point, while a decrease in pressure can increase it.
| Factor | Description |
|---|---|
| Dissolved Substances | Lower the freezing point through freezing point depression |
| Pressure | Increase in pressure decreases the freezing point, while decrease in pressure increases it |
| Ice Nucleating Agents | Facilitate the formation of ice crystals, potentially altering the freezing point |
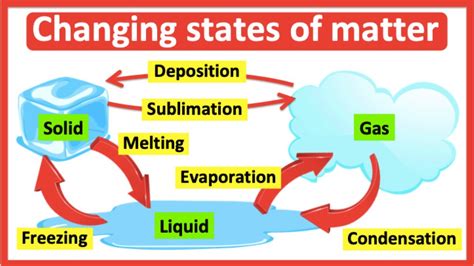
Significance in Different Fields
The water freezing point has significant implications in various fields, including chemistry, biology, and environmental science. In chemistry, understanding the freezing point is essential for the development of antifreeze materials and the preservation of chemicals. In biology, the freezing point plays a critical role in the preservation of tissues and organs for medical research and transplantation. In environmental science, the freezing point is used to model climate patterns, predict weather events, and understand the formation of glaciers and sea ice.
Applications and Research
Research into the water freezing point has numerous applications, from cryopreservation to climate modeling. Cryopreservation, the process of preserving tissues and organs at low temperatures, relies heavily on understanding the freezing point of water. Climate modeling, which aims to predict future climate patterns, also depends on accurate representations of the freezing point and its influence on global ice cover. Additionally, the development of antifreeze materials, which can prevent the freezing of water in various applications, is an active area of research.
What is the freezing point of water under standard conditions?
+The freezing point of water under standard conditions is 0°C or 32°F at standard atmospheric pressure.
How does pressure influence the freezing point of water?
+An increase in pressure can lower the freezing point of water, while a decrease in pressure can increase it.
What is the significance of the freezing point in cryopreservation?
+Cryopreservation relies heavily on understanding the freezing point of water to preserve tissues and organs at low temperatures.
In conclusion, the water freezing point is a complex and dynamic property that plays a crucial role in various scientific and engineering applications. Understanding the factors that influence the freezing point, its significance in different fields, and its applications in research is essential for advancing our knowledge of this fundamental concept. As research continues to uncover the intricacies of the water freezing point, we can expect to see significant advancements in fields like cryopreservation, climate modeling, and the development of antifreeze materials.