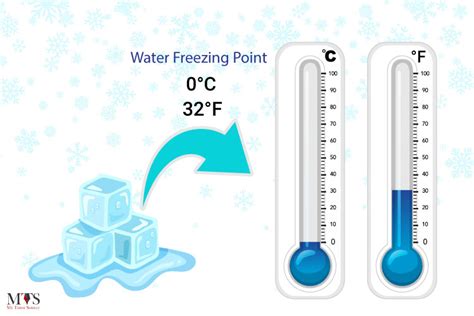
Water, the most abundant substance on Earth, has a unique property that is fundamental to our understanding of physics and chemistry. One of the most well-known properties of water is its freezing point, which is a crucial factor in various aspects of our daily lives, from weather forecasting to industrial applications. At a temperature of 32 degrees Fahrenheit (°F), water undergoes a phase transition, changing from a liquid to a solid state. This process, known as freezing, is a complex phenomenon that involves the arrangement of water molecules in a crystalline structure.
The freezing point of water is a precisely defined value, which is 32 °F or 0 degrees Celsius (°C) at standard atmospheric pressure. This temperature is a critical threshold, below which water molecules slow down and come together to form a solid crystal lattice. The freezing process is an exothermic reaction, releasing heat energy into the surroundings as the water molecules release their kinetic energy and settle into a more ordered arrangement. This phase transition is accompanied by a change in the density of water, with ice being less dense than liquid water, which is why ice floats on top of liquid water.
Key Points
- The freezing point of water is 32 °F (0 °C) at standard atmospheric pressure.
- Water undergoes a phase transition from liquid to solid at its freezing point.
- The freezing process is an exothermic reaction, releasing heat energy into the surroundings.
- Ice is less dense than liquid water, which is why it floats on top of liquid water.
- The freezing point of water is a critical factor in various aspects of our daily lives, from weather forecasting to industrial applications.
The Science Behind Water Freezing
The science behind water freezing is rooted in the molecular structure of water and the intermolecular forces that govern its behavior. Water molecules are polar, meaning they have a slightly positive charge on the hydrogen atoms and a slightly negative charge on the oxygen atom. This polarity allows water molecules to form hydrogen bonds with each other, which are weak electrostatic attractions that play a crucial role in the freezing process. As the temperature of water decreases, the kinetic energy of the molecules decreases, allowing them to come closer together and form a more ordered arrangement.
Factors Affecting the Freezing Point of Water
The freezing point of water can be affected by various factors, including pressure, dissolved substances, and the presence of impurities. For example, an increase in pressure can lower the freezing point of water, while a decrease in pressure can raise it. Dissolved substances, such as salt or sugar, can also lower the freezing point of water, a phenomenon known as freezing-point depression. Additionally, the presence of impurities, such as air bubbles or suspended particles, can affect the freezing point of water by providing nucleation sites for ice crystal formation.
| Factor | Effect on Freezing Point |
|---|---|
| Increased Pressure | Lower Freezing Point |
| Dissolved Substances | Lower Freezing Point (Freezing-Point Depression) |
| Impurities (Air Bubbles, Suspended Particles) | Affect Freezing Point by Providing Nucleation Sites |
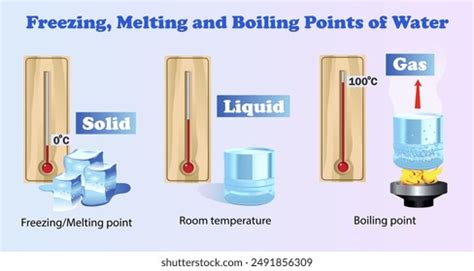
Practical Applications of Water Freezing
The freezing of water has numerous practical applications in various fields, including engineering, agriculture, and medicine. For example, the freezing of water is used in refrigeration systems to cool food and beverages, while in cryogenics, it is used to preserve biological samples and materials at extremely low temperatures. In agriculture, the freezing of water is used to protect crops from frost damage, while in medicine, it is used to preserve organs and tissues for transplantation.
In conclusion, the freezing of water at 32 °F is a complex phenomenon that involves the arrangement of water molecules in a crystalline structure. Understanding the science behind water freezing and the factors that affect its freezing point is essential for optimizing various industrial and practical applications. By recognizing the importance of water freezing, we can better appreciate the intricate mechanisms that govern the behavior of this vital substance and harness its properties to improve our daily lives.
What is the freezing point of water in Celsius?
+The freezing point of water is 0 degrees Celsius (°C) at standard atmospheric pressure.
Why does ice float on top of liquid water?
+Ice floats on top of liquid water because it is less dense than liquid water. This is due to the arrangement of water molecules in the crystalline structure of ice, which is more open and less dense than the arrangement of molecules in liquid water.
What factors can affect the freezing point of water?
+The freezing point of water can be affected by various factors, including pressure, dissolved substances, and the presence of impurities. For example, an increase in pressure can lower the freezing point of water, while a decrease in pressure can raise it. Dissolved substances, such as salt or sugar, can also lower the freezing point of water, a phenomenon known as freezing-point depression.