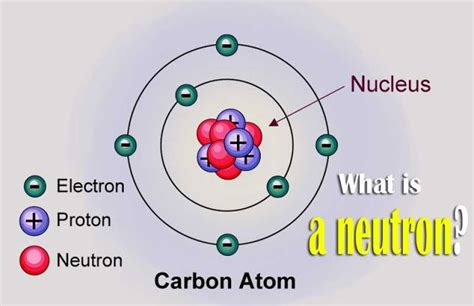
The neutron, a fundamental particle in the nucleus of an atom, has several fascinating properties. One of the most intriguing aspects of neutrons is their charge, or rather, the lack thereof. Here are five key facts about the neutron charge:
Introduction to Neutron Charge

Neutrons, along with protons, make up the nucleus of an atom. While protons have a positive charge, neutrons are known for having no charge, or being neutral. This neutrality plays a crucial role in the stability and structure of atoms. The neutron’s charge, or lack of it, is precisely zero, which distinguishes it from other subatomic particles like electrons and protons.
Historical Context of Neutron Discovery
The discovery of neutrons by James Chadwick in 1932 revolutionized the field of nuclear physics. Before this discovery, it was believed that atoms consisted only of protons and electrons. Chadwick’s experiment, which involved bombarding beryllium with alpha particles, led to the detection of a neutral particle that could penetrate thick layers of lead without being deflected by magnetic fields, characteristics that define neutrons.
| Particle | Charge |
|---|---|
| Proton | Positive (+1.602 x 10^-19 C) |
| Electron | Negative (-1.602 x 10^-19 C) |
| Neutron | No charge (0 C) |

Role of Neutrons in Atomic Stability

Neutrons play a pivotal role in maintaining the stability of the atomic nucleus. The number of neutrons in an atom’s nucleus, combined with the number of protons, determines the mass number of the atom. Variations in the number of neutrons, while keeping the proton number constant, result in different isotopes of the same element. These isotopes have the same chemical properties but differ in their physical properties, such as mass and radioactivity.
Neutron Interactions and Applications
Neutrons interact with matter primarily through the strong nuclear force, which is much stronger than electromagnetic forces but acts over a very short range. This interaction is the basis for various applications, including nuclear reactors, where neutrons induce fission reactions, and neutron scattering techniques, which are used to study the structure of materials at the atomic level.
Key Points
- Neutrons have no electric charge, making them distinct from protons and electrons.
- The discovery of neutrons by James Chadwick in 1932 was a significant milestone in nuclear physics.
- Neutrons are crucial for the stability of atomic nuclei, helping to balance the positive charge of protons.
- Variations in neutron numbers result in isotopes of elements, which have the same chemical but different physical properties.
- Neutron interactions are foundational for applications like nuclear energy and material science research.
In conclusion, the neutron charge, or its neutrality, is a fundamental aspect of physics that underpins our understanding of atomic structure and nuclear interactions. The role of neutrons in maintaining nuclear stability, their discovery, and their applications underscore their importance in the field of physics and beyond.
What is the significance of the neutron’s neutral charge?
+The neutrality of neutrons is significant because it allows them to contribute to the mass of the nucleus without contributing to its charge, thereby helping to stabilize the nucleus against the repulsive forces between protons.
How were neutrons discovered?
+Neutrons were discovered by James Chadwick in 1932 through an experiment where he bombarded beryllium with alpha particles, resulting in the emission of a neutral particle that could penetrate thick layers of lead.
What role do neutrons play in nuclear reactions?
+Neutrons play a crucial role in nuclear reactions, such as fission and fusion, by either inducing reactions or being produced as a result of these reactions. They are also essential for the operation of nuclear reactors.