Water freezing is a fundamental physical process that occurs when the temperature of water drops to a certain point, causing it to transition from a liquid to a solid state. The temperature at which water freezes is a well-established scientific fact, widely recognized as 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F) at standard atmospheric pressure. However, the concept of "5 degrees water freezes" seems to be a misunderstanding or misrepresentation of this basic principle. In reality, water does not freeze at 5 degrees above or below its freezing point under normal conditions. To clarify and provide a comprehensive understanding of water's freezing behavior, it's essential to delve into the physics behind the freezing process and explore how temperature affects the state of water.
Understanding the Freezing Point of Water
The freezing point of water is determined by the balance between the kinetic energy of its molecules and the intermolecular forces that hold them together. At temperatures above 0°C, water molecules have enough kinetic energy to overcome these forces, allowing them to move freely and maintain their liquid state. As the temperature drops to 0°C, the kinetic energy decreases to a point where the intermolecular forces can hold the molecules in a fixed, crystalline structure, causing water to freeze. This process is highly dependent on the temperature and pressure conditions. For instance, under increased pressure, water can remain in a liquid state below 0°C, a phenomenon known as supercooling, until it is disturbed and rapidly freezes.
Influence of Temperature on Water’s State
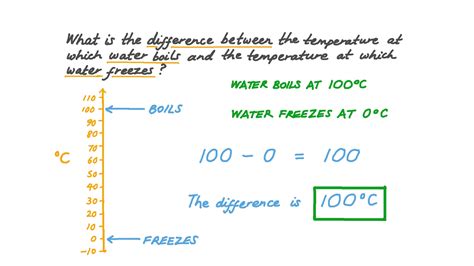
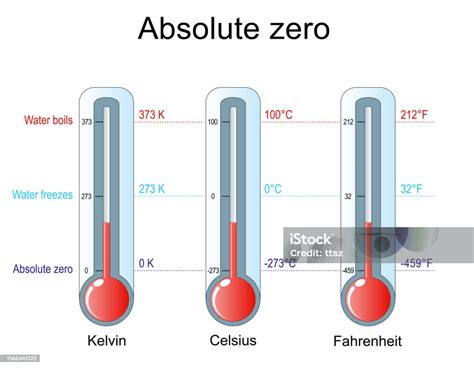
Temperature plays a crucial role in determining the state of water. At standard atmospheric pressure, water freezes at 0°C and boils at 100°C. However, these temperatures can vary slightly with changes in pressure. For example, at higher pressures, water will boil at a higher temperature and freeze at a lower temperature than at standard pressure. The concept of “5 degrees water freezes” does not align with these established scientific principles, suggesting a potential confusion with the freezing behavior of water under specific conditions or the effects of impurities and pressure on its freezing point.
| Temperature (°C) | State of Water |
|---|---|
| 0 | Freezing point, transition from liquid to solid |
| 100 | Boiling point, transition from liquid to gas |
Implications of Misunderstanding Water’s Freezing Point

Misunderstandings about the freezing point of water can have significant implications in fields such as chemistry, physics, and engineering, where the precise control of water’s state is critical. For instance, in the design of cooling systems, understanding the exact temperature at which water freezes is essential for preventing damage from ice formation. Similarly, in scientific research, accurate knowledge of water’s freezing behavior under different conditions is vital for conducting experiments and interpreting results correctly.
Practical Applications and Considerations
In practical applications, the freezing point of water is a critical factor. For example, in the construction of water pipes, materials and designs must be chosen to withstand the expansion of water as it freezes, to prevent pipes from bursting. In chemistry, the freezing point depression is used as a method for determining the molality of solutions, relying on the principle that the freezing point of a solution is lower than that of pure water. These applications underscore the importance of a clear understanding of water’s freezing behavior and the potential consequences of misconceptions about its freezing point.
Key Points
- Water freezes at 0°C (32°F) at standard atmospheric pressure, not at 5 degrees above or below this point.
- The freezing point can be influenced by pressure and the presence of impurities.
- Understanding the precise freezing point of water is crucial for various scientific, engineering, and everyday applications.
- Misconceptions about water's freezing point can have significant implications in fields where the control of water's state is critical.
- Practical applications, such as the design of cooling systems and construction of water pipes, rely on accurate knowledge of water's freezing behavior.
In conclusion, the concept of "5 degrees water freezes" is not supported by scientific evidence and contradicts the well-established freezing point of water at 0°C under standard conditions. Understanding the precise freezing point of water and the factors that influence it is essential for both theoretical knowledge and practical applications across various disciplines. By recognizing and addressing misconceptions about water's freezing behavior, we can better apply this knowledge to real-world problems and advancements.
What is the freezing point of water under standard atmospheric pressure?
+The freezing point of water under standard atmospheric pressure is 0°C (32°F).
Can the presence of impurities affect the freezing point of water?
+Yes, impurities can lower the freezing point of water, a phenomenon known as freezing point depression.
Why is understanding the freezing point of water important in practical applications?
+Understanding the freezing point of water is crucial for designing systems that involve water, such as cooling systems and water pipes, to prevent damage from freezing and to ensure efficient operation.



