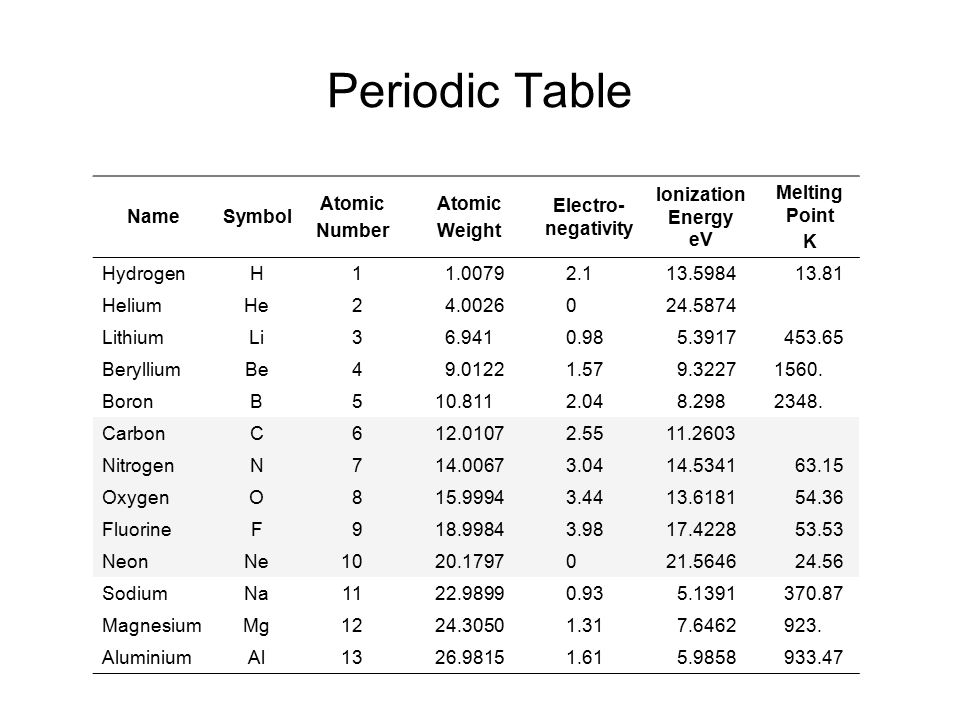
The atomic number is a fundamental concept in chemistry, representing the number of protons present in the nucleus of an atom. This value determines the chemical properties of an element and its position in the periodic table. With 118 known elements, each with its unique atomic number, understanding the atomic number is crucial for chemists, physicists, and materials scientists. The concept of atomic number was first introduced by Henry Moseley in 1913, revolutionizing the field of chemistry by providing a logical and systematic way to arrange elements based on their atomic structure rather than their atomic weights.
Historical Development of Atomic Number Concept
The development of the atomic number concept involved the contributions of several scientists over the years. Initially, Dmitri Mendeleev arranged elements in the periodic table based on their atomic weights and chemical properties. However, this arrangement had some discrepancies, which were later resolved by Moseley’s discovery that the atomic number, rather than atomic weight, was the fundamental property that determined an element’s position in the periodic table. Moseley’s work was based on X-ray spectroscopy, where he observed that the frequencies of the X-rays emitted by an element were related to its atomic number. This breakthrough led to a deeper understanding of the atomic structure and paved the way for significant advancements in chemistry and physics.
Significance of Atomic Number in Chemistry
The atomic number has profound implications in chemistry, as it dictates the number of electrons in a neutral atom, which in turn determines the chemical properties of an element. Elements with the same number of electrons in their outermost shell exhibit similar chemical behavior, a principle that underlies the periodicity of elements. The atomic number also plays a crucial role in nuclear chemistry, where it influences the stability of nuclei and the types of radioactive decay that can occur. Understanding these aspects is essential for predicting the behavior of elements under various conditions, designing new materials, and developing nuclear technologies.
| Element | Atomic Number | Number of Electrons |
|---|---|---|
| Hydrogen | 1 | 1 |
| Helium | 2 | 2 |
| Oxygen | 8 | 8 |
| Carbon | 6 | 6 |
| Uranium | 92 | 92 |
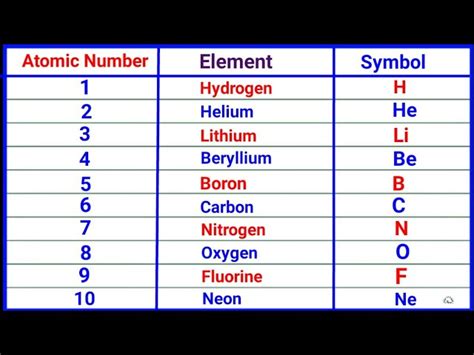
Atomic Number and Electron Configuration
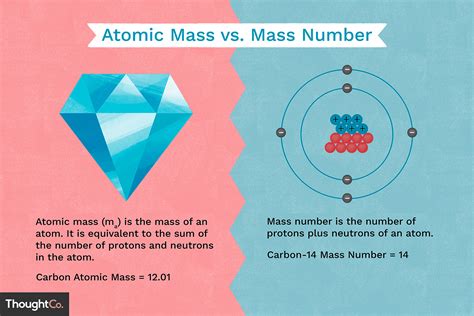
The atomic number is directly related to the electron configuration of an atom, which describes how electrons are distributed among the various atomic orbitals. The electron configuration, in turn, influences the chemical properties of an element, such as its reactivity and the types of compounds it can form. The arrangement of electrons follows the Aufbau principle and the Pauli exclusion principle, ensuring that each orbital is filled in a specific order and that no two electrons in an atom have the same set of quantum numbers, respectively. This systematic approach to understanding electron configuration is grounded in the atomic number, reinforcing its central role in chemistry.
Applications of Atomic Number in Science and Technology
The atomic number has numerous applications in science and technology. In materials science, understanding the atomic number of elements is crucial for designing materials with specific properties, such as conductivity, strength, and optical characteristics. In nuclear medicine, the atomic number of elements used in radioactive tracers and treatments is critical for their effectiveness and safety. Furthermore, the atomic number plays a key role in the development of semiconductors, which are fundamental components of modern electronics. The precise control over the atomic composition of semiconductor materials allows for the creation of devices with tailored electrical properties, underscoring the significance of the atomic number in technological innovations.
Key Points
- The atomic number is a unique identifier for each element, determining its chemical properties and position in the periodic table.
- Understanding the atomic number is essential for predicting the behavior of elements, designing new materials, and developing nuclear technologies.
- The atomic number influences the electron configuration of an atom, which in turn affects its chemical reactivity and physical properties.
- The concept of atomic number has numerous applications in materials science, nuclear medicine, and electronics.
- The atomic number was first introduced by Henry Moseley, revolutionizing the field of chemistry by providing a logical and systematic way to arrange elements based on their atomic structure.
In conclusion, the atomic number is a foundational concept in chemistry and physics, with far-reaching implications for our understanding of the elements and their properties. Its significance extends beyond the realm of pure science, influencing technological advancements and practical applications across various fields. As our knowledge of atomic structure and properties continues to evolve, the importance of the atomic number remains steadfast, serving as a cornerstone of scientific inquiry and innovation.
What is the atomic number, and why is it important?
+The atomic number is the number of protons present in the nucleus of an atom and is crucial for determining the chemical properties of an element and its position in the periodic table.
How does the atomic number relate to the electron configuration of an atom?
+The atomic number directly influences the electron configuration, as it determines the total number of electrons in a neutral atom, which in turn affects the chemical reactivity and physical properties of an element.
What are some practical applications of the atomic number in science and technology?
+The atomic number has applications in materials science, nuclear medicine, and electronics, among others, where understanding the atomic composition of materials is critical for designing and developing new technologies and treatments.