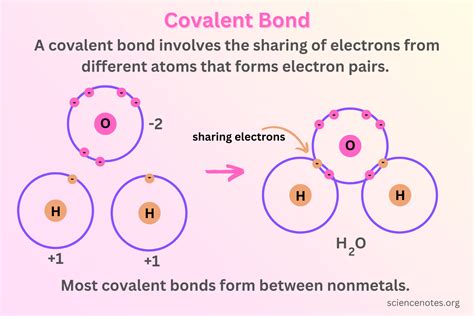
The behavior of electrons in covalent bonds is a fundamental aspect of chemistry, underlying the formation and properties of molecules. Covalent bonds are characterized by the sharing of electron pairs between atoms, leading to the formation of a stable molecular structure. The understanding of electron behavior in these bonds is crucial for explaining the chemical and physical properties of molecules. In covalent bonding, the electrons are not transferred from one atom to another, as in ionic bonds, but are shared in a way that the resulting molecule has a lower energy state than the individual atoms.
One of the key principles governing electron behavior in covalent bonds is the octet rule. This rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell, which typically consists of eight electrons. This configuration is particularly stable, mimicking the electron configuration of the noble gases. The sharing of electrons allows atoms that are not noble gases to achieve this stable configuration, thereby forming a covalent bond. The electrons in a covalent bond are distributed in a way that each atom achieves an octet, except in cases like hydrogen, which requires only two electrons to fill its outer shell.
Key Points
- The behavior of electrons in covalent bonds is governed by the principle of achieving a stable molecular structure through the sharing of electron pairs.
- The octet rule is a fundamental principle, where atoms share electrons to achieve a full outer shell of eight electrons, mimicking the noble gas configuration.
- The polarity of a covalent bond is influenced by the electronegativity difference between the atoms involved, affecting the distribution of electrons.
- Orbital hybridization is a critical concept in understanding the spatial distribution of electrons in covalent bonds, explaining the geometry of molecules.
- The study of electron behavior in covalent bonds is essential for understanding chemical reactivity, molecular properties, and the formation of compounds.
Electronegativity and Bond Polarity
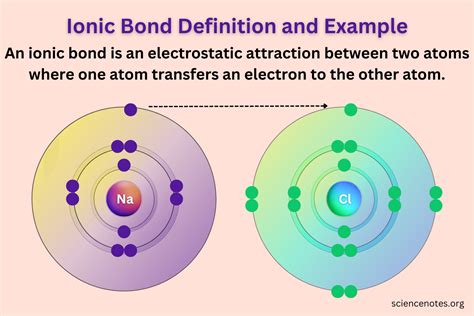
Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond. When two atoms with different electronegativities form a covalent bond, the bond is polar. The atom with higher electronegativity pulls the shared electrons closer to itself, resulting in a partial positive charge on the other atom and a partial negative charge on the more electronegative atom. This polarity affects the physical and chemical properties of the molecule, such as its boiling point, melting point, and reactivity. For example, the water molecule (H2O) has a polar covalent bond between oxygen and hydrogen due to the significant difference in electronegativity between oxygen and hydrogen, leading to its high boiling point and solvent properties.
Orbital Hybridization
Orbital hybridization is a concept used to explain the shape of molecules and the orientation of covalent bonds. In hybridization, the atomic orbitals of an atom mix to form new hybrid orbitals, which are suitable for the pairing of electrons to form chemical bonds. The number of hybrid orbitals formed and their geometry depend on the number of atomic orbitals mixed. For instance, sp3 hybridization involves the mixing of one s orbital and three p orbitals, resulting in four equivalent hybrid orbitals oriented tetrahedrally around the central atom. This concept is crucial for understanding the three-dimensional structure of molecules and the directional nature of covalent bonds.
| Hybridization Type | Geometry | Example |
|---|---|---|
| sp3 | Tetrahedral | Methane (CH4) |
| sp2 | Trigonal Planar | Ethene (C2H4) |
| sp | Linear | Ethyne (C2H2) |

Applications and Implications

The study of electron behavior in covalent bonds has numerous applications in chemistry and related fields. It provides the foundation for understanding chemical reactions, including how molecules interact and transform into products. The knowledge of bond polarity and molecular geometry is critical in drug design, where the interaction between a drug molecule and its target, such as a protein, depends on the shape and polarity of the drug molecule. Additionally, understanding covalent bonding is essential in materials science, where the properties of materials, such as strength, conductivity, and optical properties, are influenced by the types of bonds and the arrangement of atoms within the material.
In conclusion, the behavior of electrons in covalent bonds is a complex and fascinating topic that underlies the structure and properties of molecules. Through the principles of electronegativity, orbital hybridization, and the octet rule, chemists can predict and explain the behavior of electrons in covalent bonds, providing a foundation for understanding chemical reactivity and the design of new materials and drugs.
What is the primary difference between covalent and ionic bonds in terms of electron behavior?
+In covalent bonds, electrons are shared between atoms to form a stable molecule, whereas in ionic bonds, electrons are transferred from one atom to another, resulting in the formation of ions that are attracted to each other.
How does electronegativity affect the polarity of a covalent bond?
+Electronegativity affects the polarity of a covalent bond by determining how the shared electrons are distributed between the atoms. An atom with higher electronegativity pulls the shared electrons closer, creating a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom.
What role does orbital hybridization play in explaining molecular geometry?
+Orbital hybridization explains the geometry of molecules by describing how atomic orbitals mix to form hybrid orbitals that are oriented in space in a way that minimizes electron repulsion and maximizes bond strength, leading to the observed molecular shapes.