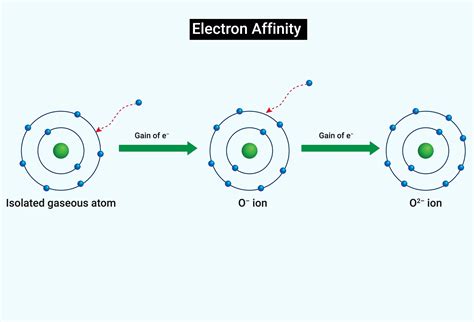
Electron affinity is a fundamental concept in chemistry, representing the energy change that occurs when an electron is added to a neutral atom or molecule. This process is crucial in understanding the reactivity of elements and their ability to form compounds. The electron affinity of an element is a measure of its tendency to attract an electron, which is significant in determining its chemical properties and behavior. In this article, we will delve into five essential facts about electron affinity, exploring its definition, trends in the periodic table, importance in chemical bonding, factors influencing its value, and its role in various chemical reactions.
Key Points
- Electron affinity is defined as the energy change associated with the addition of an electron to a neutral atom or molecule.
- The electron affinity values exhibit periodic trends, with elements in the same group of the periodic table showing similar affinities.
- Electron affinity plays a critical role in the formation of chemical bonds, particularly in ionic bonding where electrons are transferred between atoms.
- Several factors, including atomic size, electronegativity, and electron configuration, influence the electron affinity of an element.
- Understanding electron affinity is essential in predicting the reactivity of elements and the stability of their compounds, with applications in fields like materials science and catalysis.
Definition and Periodic Trends of Electron Affinity
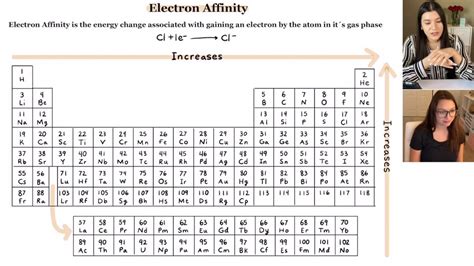
Electron affinity is quantitatively expressed as the energy change (ΔE) in the reaction: A + e⁻ → A⁻, where A is the neutral atom or molecule, and e⁻ is the electron being added. A negative value of electron affinity indicates that the addition of an electron is energetically favorable, meaning the atom or molecule has a tendency to attract electrons. Conversely, a positive value suggests that energy is required to add an electron, indicating a lower tendency to attract electrons. The periodic table exhibits trends in electron affinity, with elements in the same group (vertical column) generally showing similar electron affinities due to the same number of electron shells and similar electron configurations.
Influence of Atomic Size and Electronegativity
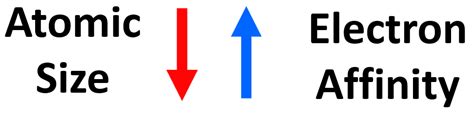
The size of an atom and its electronegativity are significant factors influencing its electron affinity. Smaller atoms tend to have higher electronegativities and thus higher electron affinities because they can attract electrons more effectively due to their smaller size and higher nuclear charge density. Conversely, larger atoms have lower electronegativities and lower electron affinities due to their larger size, which results in a lower nuclear charge density and a weaker attraction to electrons. Additionally, the electron configuration of an atom, particularly the presence of half-filled or completely filled subshells, can significantly influence its electron affinity, as these configurations are particularly stable and less likely to accept additional electrons.
| Element | Electron Affinity (kJ/mol) |
|---|---|
| Fluorine (F) | -328 |
| Chlorine (Cl) | -349 |
| Bromine (Br) | -325 |
| Iodine (I) | -295 |
Importance of Electron Affinity in Chemical Bonding
Electron affinity plays a vital role in the formation of chemical bonds, particularly in ionic bonding, where one or more electrons are transferred from one atom to another, resulting in the formation of ions with opposite charges that attract each other. The difference in electron affinity between two atoms is a key factor in determining the direction of electron transfer and the stability of the resulting ionic compound. In covalent bonding, electron affinity influences the polarity of the bond, with atoms of higher electron affinity pulling the shared electrons closer, leading to a polar covalent bond.
Applications and Implications of Electron Affinity
The understanding of electron affinity has numerous practical applications. In materials science, knowledge of electron affinity is essential for designing and optimizing the properties of semiconductors and other electronic materials. In catalysis, the electron affinity of metals and their oxides can influence their catalytic activity, affecting the efficiency of chemical reactions. Furthermore, electron affinity is critical in understanding the reactivity of atmospheric pollutants and the stability of molecules in biological systems, highlighting its significance across various disciplines.
What is electron affinity, and why is it important in chemistry?
+Electron affinity is the energy change associated with adding an electron to a neutral atom or molecule. It's crucial in understanding chemical reactivity, particularly in the formation of ionic and covalent bonds, and has implications in materials science, catalysis, and environmental chemistry.
How does electron affinity vary across the periodic table?
+Electron affinity exhibits periodic trends, generally increasing across a period from left to right due to increasing nuclear charge and decreasing atomic size, and decreasing down a group due to increasing atomic size and decreasing nuclear charge density.
What factors influence the electron affinity of an element?
+The electron affinity of an element is influenced by its atomic size, electronegativity, and electron configuration. Smaller atoms with higher electronegativities tend to have higher electron affinities. The presence of half-filled or completely filled subshells can also significantly affect an element's electron affinity.
In conclusion, electron affinity is a critical concept in chemistry that influences the chemical behavior of elements and their compounds. Understanding its definition, periodic trends, and the factors that influence its value is essential for predicting reactivity and designing new materials and reactions. The implications of electron affinity are far-reaching, from the formation of chemical bonds to applications in materials science and catalysis, underscoring its importance in both theoretical and applied chemistry.