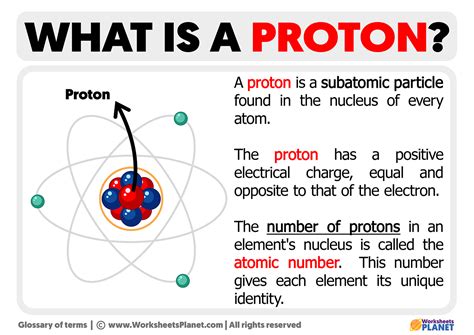
The concept of proton charge is fundamental to our understanding of the atomic structure and the behavior of matter at the subatomic level. Protons, along with neutrons, reside in the nucleus of an atom, and their positive charge plays a crucial role in determining the chemical properties of an element. The proton charge, denoted by the symbol e or sometimes e+ to distinguish it from the electron charge, is a basic constant in physics and chemistry.
Historically, the discovery of the proton and its charge can be attributed to the work of Ernest Rutherford and his famous gold foil experiment in 1909. This experiment led to the development of the nuclear model of the atom, where the positive charge is concentrated in a small nucleus, surrounded by electrons. Later, in 1919, Rutherford himself discovered the proton by bombarding nitrogen gas with alpha particles, observing that the nucleus of nitrogen emitted a particle that was identified as hydrogen nuclei, which we now know as protons.
Key Points
- The proton charge is approximately +1.602 x 10^-19 Coulombs, a fundamental constant in physics.
- Protons reside in the nucleus of an atom, contributing to its overall positive charge.
- The number of protons in an atom's nucleus determines its atomic number and, consequently, the element's identity.
- Protons and electrons have equal but opposite charges, ensuring that a neutral atom has a balanced charge.
- Understanding proton charge is crucial for explaining chemical bonding and reactions, as well as the stability of atomic nuclei.
Quantification of Proton Charge
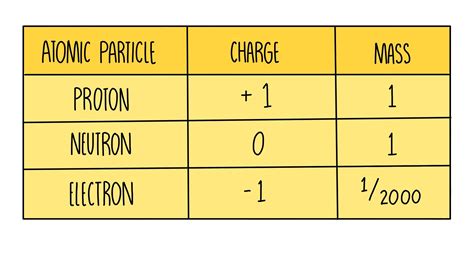
The charge of a proton is quantified as approximately +1.602 x 10^-19 Coulombs. This value is a fundamental constant in physics and is denoted by e. The quantification of charge is crucial because it allows us to understand and predict the behavior of charged particles in various physical and chemical contexts. The precision with which we know the proton charge is a testament to the advances in experimental physics and the importance of precise measurements in understanding the natural world.
Proton Charge and Atomic Structure
The proton charge plays a pivotal role in the structure of atoms. The number of protons in an atom’s nucleus determines its atomic number (Z), which in turn defines the chemical element. For instance, hydrogen has one proton, helium has two, and so on. The positive charge of the protons is balanced by the negative charge of the electrons orbiting the nucleus, resulting in a neutral atom if the number of electrons equals the number of protons. This balance is essential for the stability and chemical properties of an element.
| Element | Atomic Number (Protons) | Charge of Protons |
|---|---|---|
| Hydrogen | 1 | +1.602 x 10^-19 C |
| Helium | 2 | +3.204 x 10^-19 C |
| Oxygen | 8 | +1.2816 x 10^-18 C |
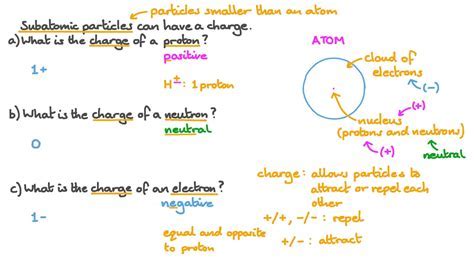
Implications of Proton Charge
The implications of proton charge are vast and far-reaching. In chemistry, the proton charge is central to understanding chemical bonding. The electrostatic attraction between the positive charge of protons in the nucleus and the negative charge of electrons leads to the formation of atomic bonds. Moreover, the transfer or sharing of electrons between atoms, which is influenced by the proton charge, underlies all chemical reactions and interactions.
In physics, the proton charge is crucial for understanding nuclear stability and radioactive decay. The balance between protons and neutrons in the nucleus affects its stability, with certain combinations leading to radioactive decay. The strong nuclear force, which binds protons and neutrons together in the nucleus, must overcome the repulsive electrostatic force between positively charged protons, highlighting the importance of proton charge in nuclear physics.
Proton Charge in Biological Contexts
While the proton charge might seem like a basic concept relevant only to atomic and nuclear physics, its implications extend into biological systems. The functioning of biological molecules, such as DNA and proteins, is heavily influenced by the chemical properties of their constituent atoms, which in turn are determined by their proton and electron configurations. For example, the acidity and basicity of amino acids in proteins, which are critical for their structure and function, are influenced by the protonation state of their side chains.
What is the significance of proton charge in atomic structure?
+The proton charge is significant because it determines the atomic number of an element, which defines its chemical properties and identity. The balance between protons and electrons leads to the formation of neutral atoms, which is crucial for the stability and reactivity of elements.
How does the proton charge influence chemical bonding?
+The proton charge attracts electrons, leading to the formation of chemical bonds. The sharing or transfer of electrons between atoms, influenced by the proton charge, underlies all chemical reactions and interactions, making it a fundamental aspect of chemistry.
What role does proton charge play in nuclear stability?
+The proton charge affects nuclear stability by contributing to the electrostatic repulsion between protons in the nucleus. The strong nuclear force must overcome this repulsion to bind the nucleus together, and the balance between protons and neutrons influences the nucleus's stability, with certain combinations leading to radioactive decay.
In conclusion, the proton charge is a fundamental aspect of physics and chemistry, influencing the structure of atoms, the properties of elements, and the behavior of matter at both atomic and nuclear levels. Its implications are far-reaching, from the chemical bonds that form molecules to the stability of atomic nuclei. Understanding the proton charge is essential for appreciating the intricate mechanisms that govern our universe, from the simplest chemical reactions to the complex processes that occur within the nucleus of an atom.