Water density is a fundamental concept in understanding the behavior of water in various environments, from oceans to lakes, and even in industrial applications. At its core, density refers to the mass per unit volume of a substance. For water, this value is approximately 1 gram per cubic centimeter (g/cm³) at standard temperature and pressure (STP) conditions, which are defined as 0°C and 1 atmosphere. However, water's density is not constant and can vary significantly with changes in temperature and pressure. This variability has profound implications for both natural phenomena and human activities.
The density of water is a critical factor in oceanography, influencing ocean currents, marine life habitats, and the global climate. For instance, the difference in density between warm and cold water drives the thermohaline circulation, a global ocean conveyor belt that plays a key role in distributing heat around the globe. Furthermore, the density of seawater, which is slightly higher than that of freshwater due to the presence of dissolved salts, affects the buoyancy of marine organisms and the formation of marine ecosystems.
Key Points
- The density of water at standard conditions is approximately 1 g/cm³.
- Temperature and pressure changes can significantly alter water's density.
- Density differences drive ocean currents and influence marine habitats.
- The density of seawater is higher than freshwater due to dissolved salts.
- Understanding water density is crucial for both environmental and industrial applications.
Variations in Water Density
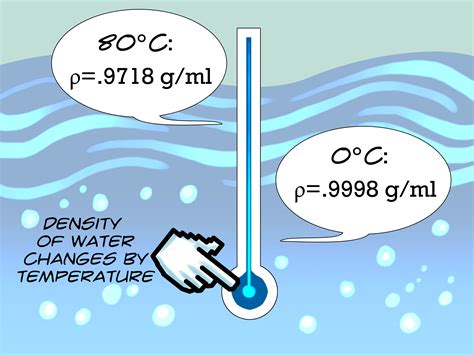
Water’s density varies inversely with temperature; as temperature increases, density decreases. This relationship is not linear but rather follows a complex curve. At 0°C, the density of freshwater is at its maximum, approximately 0.9998 g/cm³. As the temperature rises to 4°C, the density decreases slightly, reaching about 0.99997 g/cm³, and then continues to decrease more significantly as the temperature approaches the boiling point. This unique property, where water expands as it cools below 4°C, is crucial for the survival of aquatic life during winter months, as it allows ice to form on the surface of lakes and rivers while the denser, warmer water remains below, providing a habitat for fish and other organisms.
Effects of Pressure on Water Density
Pressure also affects the density of water, though to a lesser extent than temperature. An increase in pressure results in an increase in density, as the molecules are compressed into a smaller volume. However, the effect of pressure on water density is minimal at pressures encountered in most natural environments. In deep-sea environments, where pressures are significantly higher, the density of seawater increases due to compression, affecting the buoyancy of deep-sea creatures and the operation of underwater vehicles.
| Temperature (°C) | Density of Freshwater (g/cm³) |
|---|---|
| 0 | 0.9998 |
| 4 | 0.99997 |
| 20 | 0.9982 |
| 100 | 0.9584 |
Practical Applications of Water Density

The understanding and application of water density principles are vital in various industries, including engineering, where the design of dams, bridges, and other water structures relies heavily on accurate calculations of water pressure and flow. In marine engineering, the buoyancy of ships and submarines is calculated based on the principle of fluid density, ensuring safe and efficient operation. Furthermore, in water treatment and desalination plants, the density of water is a key factor in the separation and purification processes.
Challenges and Future Directions
Despite the significance of water density, there are challenges in accurately measuring and predicting its variations, especially in complex environments such as the ocean, where salinity, temperature, and pressure all play roles. Advances in sensor technology and computational models are addressing these challenges, enabling more precise predictions of ocean currents and water properties. This research has implications for climate modeling, marine conservation, and the development of sustainable water management practices.
What is the significance of water's maximum density at 4°C?
+This property allows ice to float on top of lakes and rivers, preserving aquatic habitats during winter.
How does pressure affect the density of water?
+An increase in pressure results in a slight increase in water density due to molecular compression.
What are some practical applications of understanding water density?
+Applications include the design of water structures, marine engineering, and water treatment processes.
In conclusion, the study of water density is multifaceted, encompassing not only the physical properties of water but also its implications for natural systems and human endeavors. As our understanding of water density and its variations deepens, so too does our ability to manage water resources sustainably, predict environmental changes, and innovate in fields dependent on water’s unique properties.