The freezing point of a substance is a fundamental concept in physics and chemistry, representing the temperature at which a liquid transforms into a solid. This phenomenon is crucial in various aspects of our daily lives, from the preservation of food to the understanding of natural phenomena like the formation of ice in polar regions. The study of freezing points is intricate, involving the understanding of molecular interactions, pressure, and the purity of substances. Here are five key facts about the freezing point that highlight its significance and the factors that influence it.
Key Points
- The freezing point of a substance is defined as the temperature at which the vapor pressure of the substance in its liquid phase equals the vapor pressure of the substance in its solid phase.
- The freezing point can be affected by factors such as pressure and the presence of impurities or solutes in the substance.
- Water has a freezing point of 0 degrees Celsius (32 degrees Fahrenheit) at standard atmospheric pressure, but this can change under different conditions.
- Certain substances can exhibit a phenomenon known as supercooling, where they remain in a liquid state below their freezing point without solidifying.
- Understanding the freezing point of substances is crucial for various applications, including the preservation of biological samples, the development of certain materials, and the prediction of weather patterns.
Definition and Importance of Freezing Point

The freezing point is a critical physical constant that characterizes the transition of a substance from the liquid to the solid state. It is defined as the temperature at which the solid and liquid phases of a substance are in equilibrium at a given pressure. This equilibrium is reflected in the equality of the chemical potentials of the two phases. The freezing point is an essential property for understanding the behavior of substances under different conditions and is vital in various scientific and industrial applications.
Influence of Pressure on Freezing Point
One of the key factors that can influence the freezing point of a substance is pressure. According to the Clausius-Clapeyron equation, the freezing point of a substance can change with pressure. For most substances, an increase in pressure results in an increase in the freezing point, although there are exceptions, such as water, where the freezing point decreases with increasing pressure up to a certain point. This phenomenon is utilized in certain technological applications, such as high-pressure freezing for food preservation and the study of materials under extreme conditions.
| Substance | Freezing Point at Standard Pressure | Change in Freezing Point with Pressure |
|---|---|---|
| Water | 0°C (32°F) | Decreases initially with increasing pressure |
| Carbon Dioxide | -56.6°C (-69.9°F) | Increases with increasing pressure |
| Helium | -272.2°C (-458°F) | Has a unique phase diagram with no solid phase at low pressures |
Impurities and Freezing Point Depression
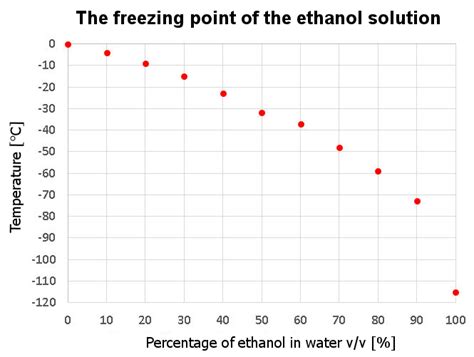
The presence of impurities or solutes in a substance can significantly affect its freezing point. This phenomenon is known as freezing point depression. When a solute is added to a solvent, it disrupts the formation of a crystal lattice structure in the solvent, requiring a lower temperature for the solution to freeze. This principle is utilized in various applications, including the preservation of food through the addition of salt or sugar, which lowers the freezing point of water and prevents the growth of ice crystals. Freezing point depression is also a method used to determine the molecular weight of a solute, based on the extent to which it depresses the freezing point of the solvent.
Supercooling and Its Implications
Supercooling is a phenomenon where a liquid remains in its liquid state below its freezing point without solidifying. This metastable state can occur when a liquid is cooled slowly and carefully to avoid nucleation sites that could initiate crystallization. Supercooling is significant in both natural and industrial contexts. For example, the supercooling of water droplets in clouds is crucial for the formation of certain types of clouds and precipitation. In industrial applications, supercooling is used in the production of glassy materials and in cryopreservation techniques.
In conclusion, the freezing point of a substance is a fundamental property that is influenced by various factors, including pressure and the presence of impurities. Understanding these factors and the phenomena associated with freezing points, such as supercooling and freezing point depression, is crucial for advancing our knowledge in physics, chemistry, and materials science, and for developing new technologies and applications.
What is the significance of the freezing point in daily life?
+The freezing point is significant in daily life for applications such as food preservation, understanding weather patterns, and the development of materials. It plays a crucial role in our ability to store food safely and predict weather conditions.
How does pressure affect the freezing point of substances?
+Pressure can either increase or decrease the freezing point of substances, depending on the substance. For most substances, an increase in pressure results in an increase in the freezing point. However, water is an exception, where the freezing point initially decreases with increasing pressure.
What is supercooling, and why is it important?
+Supercooling is a phenomenon where a liquid remains in its liquid state below its freezing point without solidifying. It is important in both natural and industrial contexts, such as cloud formation and the production of certain materials.