The concept of density is a fundamental aspect of physics and chemistry, and it is defined as the mass of an object per unit volume. One of the most commonly used units to express density is grams per cubic centimeter (g/cm³). This unit is particularly useful when dealing with solids and liquids, as it provides a straightforward way to compare the mass of different substances in relation to their volume.
Key Points
- The density of a substance is defined as its mass per unit volume, typically expressed in grams per cubic centimeter (g/cm³).
- Density is an intensive property, meaning it does not depend on the size or amount of the substance.
- The density of a substance can be used to identify it, as different substances have unique densities.
- Density is also important in various industrial and scientific applications, such as calculating the volume of a substance given its mass and density.
- Understanding density is crucial in fields like chemistry, physics, and engineering, where it is used to describe the properties of materials and predict their behavior under different conditions.
Understanding Density and Its Importance
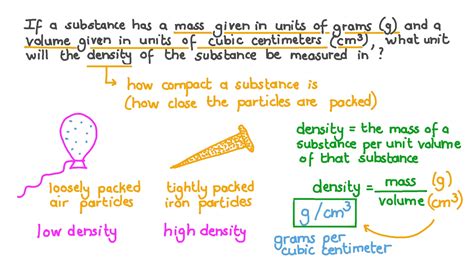
Density is a physical property that is inherent to every substance, and it is measured by dividing the mass of the substance by its volume. The formula for density is ρ = m/V, where ρ is the density, m is the mass, and V is the volume. When using grams per cubic centimeter as the unit of measurement, the density of water, for example, is approximately 1 g/cm³ at room temperature. This means that one cubic centimeter (or one milliliter) of water has a mass of one gram.
Applications of Density in Science and Industry
The concept of density has numerous practical applications across various fields. In chemistry, density is used to identify substances and determine their purity. For instance, the density of a sample can be compared to the known density of a pure substance to assess its authenticity. In physics, density is crucial for understanding the behavior of materials under different conditions, such as pressure and temperature. Engineers also rely on density calculations to design and optimize systems, ensuring that materials are used efficiently and safely.
| Substance | Density (g/cm³) |
|---|---|
| Water | 1 |
| Mercury | 13.546 |
| Lead | 11.34 |
| Air | 0.0012 |
Calculating Density and Its Practical Applications

To calculate the density of a substance, one needs to know its mass and volume. The mass can be measured using a balance, and the volume can be determined using a variety of methods depending on the substance’s state (solid, liquid, or gas). For solids and liquids, a graduated cylinder or a pycnometer can be used. Once the mass and volume are known, the density can be calculated using the formula ρ = m/V. This calculation is essential in many practical applications, such as determining the amount of a substance needed for a specific application or understanding how materials will interact in a given environment.
Examples of Density Calculations
Consider a piece of metal with a mass of 200 grams and a volume of 20 cubic centimeters. The density of this metal can be calculated as ρ = 200 g / 20 cm³ = 10 g/cm³. This means the metal is denser than water but less dense than lead. Such calculations are vital in engineering for selecting materials that meet specific criteria, such as strength, durability, and weight.
What is the significance of density in chemistry?
+Density is significant in chemistry because it allows for the identification of substances, determination of their purity, and calculation of the amount of substance needed for reactions. It's also crucial for understanding the physical properties and behavior of chemicals.
How does temperature affect the density of a substance?
+Temperature can affect the density of a substance by causing it to expand or contract. Most substances expand when heated and contract when cooled, which changes their volume and, consequently, their density. This effect is more pronounced in gases than in liquids or solids.
What are some common units of density besides grams per cubic centimeter?
+Besides grams per cubic centimeter, density can also be expressed in units such as kilograms per cubic meter (kg/m³), pounds per cubic foot (lb/ft³), and grams per milliliter (g/mL). The choice of unit depends on the context and the substances being compared.
In conclusion, the concept of density, particularly when expressed in grams per cubic centimeter, is fundamental to understanding the physical properties of substances and their behavior under various conditions. Its applications span across chemistry, physics, engineering, and many industrial processes, making it a crucial piece of knowledge for professionals and scholars alike. By grasping the principles of density and how to calculate and apply it, individuals can better comprehend the intricate relationships between mass, volume, and the properties of materials, ultimately contributing to advancements in science and technology.