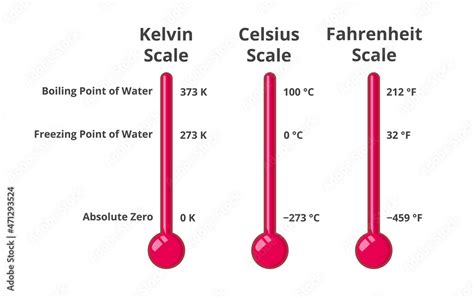
The water freezing point is a fundamental physical constant that has significant implications in various fields, including chemistry, physics, and engineering. At standard atmospheric pressure, the freezing point of water is precisely 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F). This temperature represents the point at which water undergoes a phase transition from a liquid to a solid state, resulting in the formation of ice. Understanding the water freezing point is crucial in a wide range of applications, from climate modeling and weather forecasting to the design of heating and cooling systems.
Key Points
- The water freezing point is 0°C or 32°F at standard atmospheric pressure.
- The freezing point is a critical parameter in climate modeling and weather forecasting.
- Changes in the freezing point can have significant effects on the environment and ecosystems.
- The freezing point of water can be affected by various factors, including pressure, dissolved substances, and temperature fluctuations.
- Understanding the water freezing point is essential for the design and operation of heating and cooling systems.
Factors Affecting the Water Freezing Point

The water freezing point is influenced by several factors, including pressure, dissolved substances, and temperature fluctuations. For example, an increase in pressure can lower the freezing point of water, a phenomenon known as pressure melting point. This effect is significant in high-pressure environments, such as those found in deep-sea ice cores or high-altitude glaciers. Conversely, the presence of dissolved substances, such as salts or sugars, can raise the freezing point of water, a process known as freezing-point depression. This effect is crucial in the production of antifreeze solutions and in the preservation of food products.
Pressure Melting Point
The pressure melting point is the temperature at which ice melts under a given pressure. At standard atmospheric pressure, the pressure melting point is equivalent to the freezing point of water. However, as pressure increases, the melting point of ice decreases, resulting in a lower freezing point. This effect is significant in high-pressure environments, where the freezing point of water can be lowered by as much as 0.0072°C per atmosphere. For example, at a pressure of 1000 atmospheres, the freezing point of water would be approximately -7.2°C.
| Pressure (atm) | Freezing Point (°C) |
|---|---|
| 1 | 0 |
| 100 | -0.72 |
| 1000 | -7.2 |
Applications of the Water Freezing Point
The water freezing point has numerous applications in various fields, including climate modeling, weather forecasting, and the design of heating and cooling systems. For example, understanding the freezing point of water is crucial in predicting the formation of ice in clouds, which can have significant effects on the Earth’s energy balance. Additionally, the freezing point of water is used as a reference point in the design of thermometers and other temperature-measuring devices.
Climate Modeling
Climate models rely heavily on the accurate representation of the water freezing point to predict the formation of ice in clouds, the growth of sea ice, and the melting of glaciers. Small changes in the freezing point can have significant effects on the climate system, and therefore, it is essential to accurately represent this parameter in climate models. For example, a study by the Intergovernmental Panel on Climate Change (IPCC) found that a 1°C change in the freezing point of water could result in a 10% change in the formation of ice in clouds.
What is the freezing point of water at high pressures?
+The freezing point of water at high pressures is lower than 0°C. For example, at a pressure of 1000 atmospheres, the freezing point of water is approximately -7.2°C.
How does the presence of dissolved substances affect the freezing point of water?
+The presence of dissolved substances can raise the freezing point of water, a process known as freezing-point depression. This effect is crucial in the production of antifreeze solutions and in the preservation of food products.
What is the significance of the water freezing point in climate modeling?
+The water freezing point is a critical parameter in climate modeling, as small changes in this parameter can have significant effects on the climate system. Accurate representation of the freezing point is essential for predicting the formation of ice in clouds, the growth of sea ice, and the melting of glaciers.
In conclusion, the water freezing point is a fundamental physical constant with significant implications in various fields. Understanding the factors that affect the freezing point, such as pressure and dissolved substances, is crucial for a wide range of applications, from climate modeling and weather forecasting to the design of heating and cooling systems. By recognizing the importance of the water freezing point, we can better appreciate the complex interactions between the atmosphere, oceans, and land surfaces that shape our planet’s climate and ecosystems.