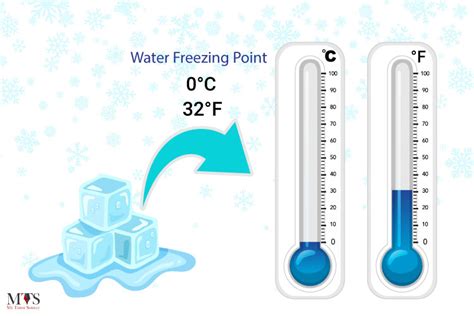
The concept of freeze temperature, often simply referred to as "freeze temp," is a critical parameter in various scientific and engineering disciplines, including physics, chemistry, biology, and materials science. It denotes the temperature at which a substance changes its state from liquid to solid, a process known as freezing. This temperature is specific to each substance and is a fundamental physical constant that characterizes the thermodynamic properties of materials.
Understanding Freeze Temperature

Freeze temperature is closely related to the melting point of a substance, which is the temperature at which a solid changes state to become a liquid. For most substances, the freezing and melting points are the same, occurring at a specific temperature where the liquid and solid phases of the substance are in equilibrium. However, under certain conditions, such as high pressure or in the presence of impurities, these temperatures can differ, a phenomenon known as supercooling or superheating.
Factors Influencing Freeze Temperature
The freeze temperature of a substance can be influenced by several factors, including pressure, the presence of impurities or solutes, and the size and shape of the particles or molecules composing the substance. For example, the freezing point of seawater is lower than that of pure water due to the dissolved salts, a phenomenon utilized in the process of desalination. Similarly, the freezing point of a solution can be lowered by the addition of antifreeze substances, which is the principle behind the use of antifreeze in automotive cooling systems.
| Substance | Freeze Temperature (°C) |
|---|---|
| Pure Water | 0 |
| Seawater | -1.8 to -3.0 |
| Ethanol (95% solution) | -114 |
| Glycerin | 17 |

Key Points
- The freeze temperature is the temperature at which a substance changes from a liquid to a solid state.
- This temperature is specific to each substance and is influenced by factors such as pressure and the presence of impurities.
- Understanding freeze temperatures is crucial in various scientific and engineering applications.
- The freeze temperature can be altered by the addition of solutes or antifreeze substances.
- Accurate knowledge of freeze temperatures is essential for preserving biological samples, developing materials, and understanding natural phenomena.
Applications of Freeze Temperature

The concept of freeze temperature has numerous practical applications across different fields. In cryogenics, the freezing temperatures of gases are critical for their storage and transportation. In biology, the preservation of tissues and cells often requires careful control of temperatures around the freezing point of water to maintain cellular integrity. In engineering, materials are selected based on their thermal properties, including their freeze temperatures, to ensure they can withstand environmental conditions.
Cryopreservation and Biological Applications
Cryopreservation, the process of preserving cells, tissues, or other biological materials at very low temperatures, relies heavily on the understanding of freeze temperatures. The goal is to slow down chemical reactions and metabolic processes to preserve the integrity of the biological material. This technique is used in medicine for preserving organs for transplantation, in reproductive medicine for preserving embryos and sperm, and in research for preserving cell lines and biological samples.
The freeze temperature also plays a crucial role in the preservation of food. Freezing is one of the most effective ways to preserve food by preventing the growth of microorganisms and reducing the rate of chemical reactions that lead to spoilage. The specific freeze temperature required can vary depending on the type of food, its water content, and the desired storage duration.
What is the significance of freeze temperature in daily life?
+The freeze temperature is significant in daily life as it affects how we preserve food, develop materials for construction and other applications, and understand natural phenomena such as weather patterns and the water cycle.
How does pressure affect the freeze temperature of a substance?
+Pressure can influence the freeze temperature of a substance. Generally, an increase in pressure will increase the freezing point, but the effect can vary depending on the substance and the magnitude of the pressure change.
What is the difference between the freezing point and the melting point of a substance?
+For most pure substances, the freezing and melting points are the same, representing the temperature at which the substance changes state between solid and liquid. However, in the presence of impurities or under different pressure conditions, these temperatures can differ.
In conclusion, the concept of freeze temperature is fundamental in understanding the physical properties of substances and has far-reaching implications across various disciplines. From the preservation of biological samples to the development of materials and the understanding of natural phenomena, the freeze temperature plays a critical role. As research and technology continue to advance, the significance of freeze temperature will only continue to grow, offering new insights into the behavior of materials at the molecular and atomic levels.