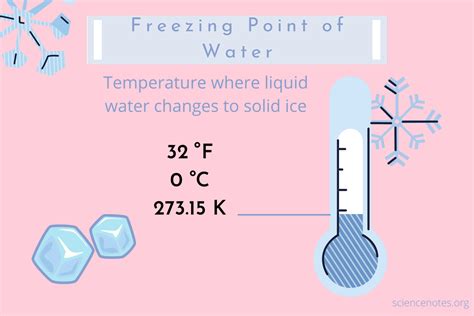
Water, the essential component of our planet, undergoes a fascinating transformation when its temperature drops to a specific point. At 32 degrees Fahrenheit (°F), water freezes, transitioning from its liquid state to a solid state, commonly known as ice. This phenomenon is a fundamental aspect of physics and chemistry, influencing various natural processes and human activities. The freezing point of water is a critical parameter in understanding weather patterns, climate, and the water cycle. Furthermore, it has significant implications for fields such as engineering, agriculture, and environmental science.
The process of water freezing is intricate, involving the arrangement of water molecules into a crystalline structure. As the temperature cools, the kinetic energy of the water molecules decreases, allowing them to form hydrogen bonds with neighboring molecules. This bonding process leads to the creation of a rigid, three-dimensional lattice structure, characteristic of ice. The freezing point of 32 °F is the temperature at which the molecules have sufficient energy to break these bonds, marking the boundary between the liquid and solid states. Understanding this transition is crucial for predicting and managing various environmental and industrial processes.
Key Points
- The freezing point of water is 32 degrees Fahrenheit (°F) or 0 degrees Celsius (°C).
- The transition from liquid to solid state is driven by the formation of hydrogen bonds between water molecules.
- Understanding the freezing point of water is essential for various fields, including meteorology, agriculture, and engineering.
- The crystalline structure of ice influences its physical properties, such as density and optical characteristics.
- Accurate prediction of freezing temperatures is critical for managing natural resources, predicting weather patterns, and designing infrastructure.
Scientific Principles Behind Freezing

The scientific principles underlying the freezing of water are rooted in thermodynamics and molecular chemistry. The temperature at which water freezes is determined by the balance between the kinetic energy of the molecules and the potential energy associated with the formation of hydrogen bonds. As the temperature decreases, the kinetic energy of the molecules decreases, allowing the potential energy of the hydrogen bonds to dominate, leading to the formation of a crystalline lattice. This process is highly dependent on the purity of the water and the presence of nucleation sites, which can influence the freezing point.
Influence of Impurities and Pressure
The presence of impurities in water can significantly affect its freezing point. For instance, seawater, which contains a high concentration of dissolved salts, freezes at a lower temperature than pure water, typically around 28.4 °F. This phenomenon, known as freezing point depression, is a colligative property of solutions and is critical in understanding oceanic processes and the formation of sea ice. Additionally, pressure can influence the freezing point of water, with higher pressures leading to a higher freezing point. This relationship is essential in understanding geological processes, such as the formation of glaciers and ice caps.
| Property | Value |
|---|---|
| Freezing Point of Pure Water | 32 °F (0 °C) |
| Freezing Point of Seawater | Approximately 28.4 °F (-2 °C) |
| Effect of Pressure on Freezing Point | Increases with increasing pressure |

Applications and Implications
The knowledge of water’s freezing point has numerous practical applications across various fields. In engineering, it is essential for designing water supply systems, HVAC systems, and infrastructure that can withstand freezing temperatures. In agriculture, understanding the freezing point of water is critical for managing crop protection and irrigation systems. Furthermore, the freezing point of water plays a pivotal role in meteorology, influencing weather forecasting, especially in predicting frost events and ice formation, which can have significant impacts on transportation, agriculture, and daily life.
The implications of water freezing at 32 °F extend beyond these practical applications, influencing our understanding of Earth's climate system. The formation of ice in polar regions and at high altitudes plays a crucial role in regulating Earth's energy balance, influencing global temperature patterns, and affecting sea levels. Moreover, changes in the freezing point of water due to climate change can have profound effects on ecosystems, biodiversity, and human societies, making the study of water's freezing behavior a critical area of research in the context of global environmental change.
Future Perspectives and Research Directions
As our understanding of the complex interactions within the Earth’s system evolves, the study of water’s freezing point remains a vibrant area of research. Future studies are likely to focus on the impacts of climate change on ice formation and melting patterns, the development of more accurate models for predicting freezing temperatures, and the exploration of new materials and technologies that can manipulate the freezing point of water for various applications. Furthermore, interdisciplinary research combining insights from physics, chemistry, biology, and engineering will be essential for addressing the challenges and opportunities arising from the freezing of water in different contexts.
What is the freezing point of pure water?
+The freezing point of pure water is 32 degrees Fahrenheit (°F) or 0 degrees Celsius (°C).
How does the presence of impurities affect the freezing point of water?
+The presence of impurities in water can lower its freezing point. For example, seawater freezes at a temperature lower than pure water due to the dissolved salts it contains.
What are the practical applications of understanding the freezing point of water?
+Understanding the freezing point of water has applications in engineering, agriculture, and meteorology. It is essential for designing infrastructure, managing crop protection, and predicting weather patterns.
In conclusion, the freezing of water at 32 degrees Fahrenheit is a fundamental phenomenon with profound implications for our understanding of natural processes and the management of human activities. Through continued research and the application of scientific principles, we can better appreciate the complexity of water’s behavior and its role in shaping our planet’s climate and ecosystems. By integrating knowledge from various disciplines, we can address the challenges posed by the freezing of water and harness its potential to create innovative solutions for a sustainable future.