The process of ion release is a fundamental concept in chemistry, particularly in the context of ionic compounds and their interactions with water. When an ionic compound dissolves in water, it breaks down into its constituent ions. For instance, consider a scenario where five ions are released from a compound. This could involve a combination of cations and anions, depending on the chemical composition of the compound. To understand this process, it's essential to delve into the basics of ionic bonding and dissolution.
Understanding Ionic Compounds and Ion Release
Ionic compounds are formed when one or more electrons are transferred between atoms, resulting in a chemical bond. This transfer leads to the formation of ions with opposite charges, which are then attracted to each other. The compound remains neutral overall, but it consists of positively charged cations and negatively charged anions. When such a compound comes into contact with water, a polar solvent, the ions are pulled away from each other by the water molecules. This process is known as dissolution, and it results in the release of ions into the solution.
The Role of Water in Ion Release
Water plays a crucial role in the dissolution of ionic compounds. Its polar nature allows it to interact with both the cations and anions of the compound, effectively pulling them apart. The oxygen atom in a water molecule has a partial negative charge, while the hydrogen atoms have a partial positive charge. This polarity enables water molecules to surround and solvate the ions, reducing the attraction between them and facilitating their release into the solution. For a compound to release five ions, it would likely involve a combination of monoatomic and polyatomic ions, depending on the specific chemical formula of the compound.
| Ion Type | Charge | Example |
|---|---|---|
| Cation | Positive | Sodium (Na+) |
| Anion | Negative | Chloride (Cl-) |
| Polyatomic Cation | Positive | Ammonium (NH4+) |
| Polyatomic Anion | Negative | Carbonate (CO32-) |
| Complex Ion | Varying | Copper(II) chloride (CuCl42-) |
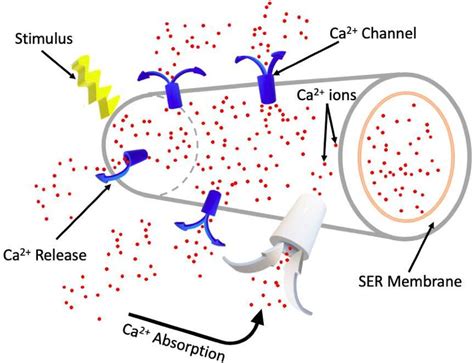
Key Factors Influencing Ion Release
Several factors can influence the release of ions from an ionic compound, including the solubility of the compound, the temperature of the solution, and the concentration of the solution. Generally, increasing the temperature or using a more polar solvent can enhance the dissolution process, leading to a more complete release of ions. However, the specific characteristics of the compound itself, such as its lattice energy and the size of its ions, also play significant roles in determining how easily it dissolves and releases its constituent ions.
Applications of Ion Release
The release of ions has numerous practical applications across various fields, including chemistry, biology, and environmental science. For instance, understanding how ions are released from compounds is crucial in the development of pharmaceuticals, where the solubility and bioavailability of drugs can significantly impact their effectiveness. Similarly, in environmental science, the release of ions from pollutants can affect water quality and have implications for aquatic life.
Key Points
- The release of ions from ionic compounds is fundamental to understanding chemical reactions and processes.
- Water's polar nature is crucial for the dissolution of ionic compounds and the release of ions.
- The characteristics of the compound, such as its solubility and lattice energy, influence the release of ions.
- Temperature and solvent polarity are key factors that can enhance the dissolution process and ion release.
- Understanding ion release has practical applications in fields like pharmaceuticals and environmental science.
In conclusion, the process of ion release from ionic compounds is a complex phenomenon influenced by various factors, including the nature of the compound, the solvent used, and environmental conditions. By grasping the principles behind ion release, scientists and researchers can better understand and predict the behavior of compounds in different solutions, leading to advancements in multiple fields of science and technology.
What is the primary factor that influences the release of ions from an ionic compound?
+The primary factor is the solubility of the compound in the solvent, which is influenced by the compound’s lattice energy and the solvent’s polarity.
How does the polarity of water contribute to the release of ions?
+Water’s polarity allows it to interact with both cations and anions, pulling them apart and facilitating their release into the solution.
What are some practical applications of understanding ion release?
+Understanding ion release has applications in the development of pharmaceuticals, environmental science, and various chemical industries, where the solubility and bioavailability of compounds are critical.