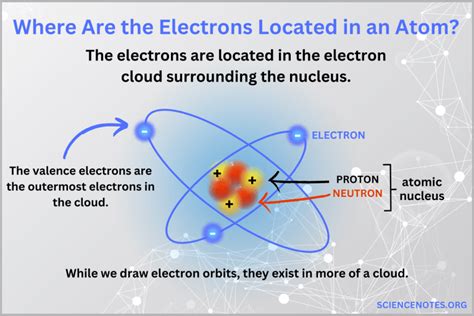
The concept of electrons and their locations is a fundamental aspect of understanding the structure of atoms, which in turn, is crucial for comprehending the principles of chemistry and physics. Electrons are subatomic particles that carry a negative charge and orbit the nucleus of an atom. The location of electrons within an atom is not random but follows specific patterns and rules, primarily described by the quantum mechanics and the electron cloud model. Here, we'll explore five key places or regions where electrons are located within an atom, which are essential for understanding chemical bonding, atomic structure, and the periodic properties of elements.
Understanding Electron Locations: An Overview
The placement of electrons in an atom is crucial for determining its chemical properties, including how it reacts with other atoms to form molecules. The atomic model, which includes the nucleus surrounded by electrons, is a cornerstone of modern chemistry. Electrons are found in various regions around the nucleus, known as electron shells or energy levels, and their arrangement is governed by the principles of quantum mechanics. Each shell can hold a specific maximum number of electrons, and the electrons in each shell are characterized by their energy level, with electrons in outer shells having higher energy than those in inner shells.
1. S Orbitals
S orbitals are spherical in shape and are the first type of orbital that electrons occupy in an atom. They are found in every energy level (or shell) and can hold up to two electrons. The s orbitals are symmetrical around the nucleus, meaning the probability of finding an electron is the same in all directions. This symmetry is crucial for understanding the chemical properties of atoms, as it influences how atoms interact with each other. For example, hydrogen and helium, the first two elements in the periodic table, have electrons in the 1s orbital, illustrating the fundamental role of s orbitals in atomic structure.
2. P Orbitals
P orbitals are dumbbell-shaped and start to appear in the second energy level of an atom. Each energy level from the second onwards has three p orbitals, which are oriented at right angles to each other. These orbitals can hold up to six electrons in total, with each orbital holding up to two electrons. The p orbitals play a significant role in the formation of chemical bonds, particularly in molecules where atoms share electrons to achieve a stable configuration. The directionality of p orbitals is essential for understanding the geometry of molecules, as seen in the VSEPR theory.
3. D Orbitals
D orbitals are more complex, with four distinct shapes, and they start appearing in the third energy level of an atom. These orbitals can hold up to ten electrons and are involved in the chemical bonding of transition metals. The d orbitals are crucial for understanding the properties of metals, including their ability to form ions and their role in catalysis. The geometry of d orbitals, with their specific orientation in space, influences the coordination chemistry of transition metals, which is vital for their applications in fields like materials science and biochemistry.
4. F Orbitals
F orbitals are the most complex, with six different shapes, and they start appearing in the fourth energy level of an atom. These orbitals can hold up to fourteen electrons and are involved in the chemical bonding of lanthanides and actinides, also known as the inner transition metals. The f orbitals are significant for understanding the unique properties of these elements, including their magnetic behaviors and their role in advanced technologies like superconductors and phosphors. The specific arrangement of f orbitals influences the spectroscopic properties of these elements, which are used in applications like lasers and optical materials.
5. Valence Shell
The valence shell is the outermost energy level of an atom that contains electrons. These electrons are known as valence electrons and are involved in chemical bonding. The number of electrons in the valence shell determines the chemical properties of an element, including its reactivity and the types of bonds it can form. The valence shell electrons are crucial for understanding the periodic trends in the periodic table, where elements with similar valence shell configurations exhibit similar chemical behaviors. For example, the noble gases have a full valence shell, which makes them chemically inert, while elements like carbon, nitrogen, and oxygen, with partially filled valence shells, are highly reactive and form the basis of organic chemistry.
| Orbital Type | Maximum Electrons | Energy Level |
|---|---|---|
| S Orbital | 2 | All energy levels |
| P Orbital | 6 | Second and onwards |
| D Orbital | 10 | Third and onwards |
| F Orbital | 14 | Fourth and onwards |
| Valence Shell | Varying | Outermost energy level |
Key Points
- Electrons are located in specific regions around the nucleus, known as electron shells or energy levels.
- S, P, D, and F orbitals have distinct shapes and capacities for holding electrons, influencing chemical bonding and properties.
- The valence shell, with its valence electrons, is crucial for determining an element's chemical reactivity and bonding capabilities.
- Understanding electron locations is essential for grasping periodic trends, chemical reactions, and the formation of molecules.
- The arrangement of electrons underlies the unique properties of elements and their applications in various technologies.
In conclusion, the locations of electrons within an atom, as described by the various orbitals and the valence shell, are fundamental to understanding the principles of chemistry and physics. This knowledge not only explains the chemical properties of elements and their reactions but also forms the basis for innovations in materials, energy, and pharmaceuticals, among other fields. The precise arrangement of electrons, governed by quantum mechanics, is a testament to the intricate and beautiful structure of matter at its most basic level.
What determines the maximum number of electrons in an orbital?
+The maximum number of electrons in an orbital is determined by the type of orbital (s, p, d, f) and the energy level it occupies. Each type of orbital has a specific capacity: s orbitals can hold up to 2 electrons, p orbitals up to 6, d orbitals up to 10, and f orbitals up to 14.
How do electrons in the valence shell influence chemical reactivity?
+Electrons in the valence shell are crucial for determining an element’s chemical reactivity. The number of valence electrons and how they are arranged influence the types of chemical bonds an atom can form and its reactivity with other atoms. A full valence shell, as seen in noble gases, indicates low reactivity, while a partially filled valence shell, as in many metals and nonmetals, indicates higher reactivity.
What is the significance of understanding electron locations in atoms?
+Understanding electron locations is vital for grasping the fundamental principles of chemistry and physics, including chemical bonding, molecular structure, and the periodic properties of elements. This knowledge underpins advancements in materials science, energy technologies, pharmaceuticals, and more, by explaining the chemical and physical properties of substances at the atomic and molecular level.