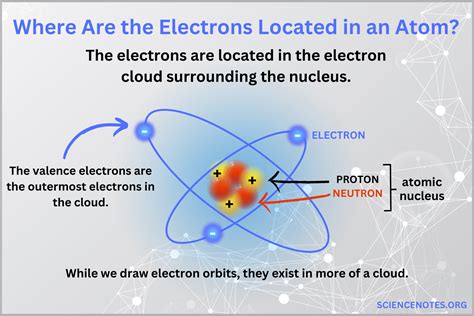
The location of electrons in an atom is a fundamental concept in chemistry and physics, as it determines the chemical properties and behavior of elements. At the heart of an atom is the nucleus, which contains protons and neutrons, surrounded by a cloud of electrons. The electrons occupy specific regions around the nucleus, known as electron shells or energy levels, which are characterized by their energy, shape, and orientation.
The arrangement of electrons in an atom is described by the electron configuration, which is a shorthand notation that indicates the number of electrons in each energy level and the type of orbital they occupy. The electron configuration is typically written in the format of [noble gas core] + [valence electrons], where the noble gas core represents the inner energy levels that are fully occupied, and the valence electrons represent the outermost energy level that is partially occupied.
Electron Shells and Energy Levels
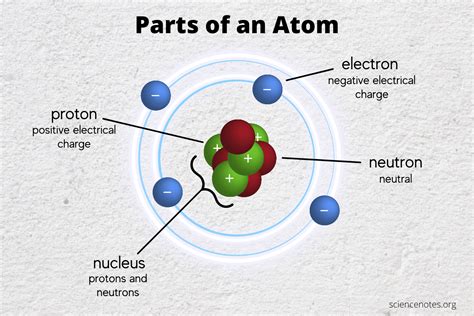
Electron shells are the regions around the nucleus where electrons are most likely to be found. Each shell has a specific energy level, and electrons in a given shell have a specific range of energies. The energy levels are typically labeled with the principal quantum number (n), which can take on values of 1, 2, 3, and so on. The first energy level (n = 1) is the closest to the nucleus and has the lowest energy, while the second energy level (n = 2) is farther away and has a higher energy.
The electron shells are further divided into subshells, which are characterized by the azimuthal quantum number (l). The subshells are labeled with letters (s, p, d, f), and each subshell has a specific shape and orientation. The s subshell is spherical in shape, while the p subshell is dumbbell-shaped, and the d and f subshells have more complex shapes.
Electron Configuration Notation
The electron configuration notation is a shorthand way of describing the arrangement of electrons in an atom. The notation typically includes the principal quantum number (n), the subshell label (s, p, d, f), and the number of electrons in each subshell. For example, the electron configuration of carbon is 1s² 2s² 2p², which indicates that the first energy level (n = 1) has two electrons in the s subshell, and the second energy level (n = 2) has two electrons in the s subshell and two electrons in the p subshell.
| Energy Level | Subshell | Electron Capacity |
|---|---|---|
| 1 | s | 2 |
| 2 | s | 2 |
| 2 | p | 6 |
| 3 | s | 2 |
| 3 | p | 6 |
| 3 | d | 10 |
Key Points
- The location of electrons in an atom determines its chemical properties and behavior.
- Electron shells are the regions around the nucleus where electrons are most likely to be found.
- Each shell has a specific energy level, and electrons in a given shell have a specific range of energies.
- The electron configuration notation is a shorthand way of describing the arrangement of electrons in an atom.
- Understanding the electron configuration is crucial in predicting an atom's chemical behavior and reactivity.
Quantum Mechanics and Electron Location
According to the principles of quantum mechanics, electrons do not have a definite position in space but instead exist as a probability distribution around the nucleus. This probability distribution is described by the wave function, which is a mathematical function that encodes the information about the electron’s position and energy. The wave function can be used to calculate the probability of finding an electron within a given region of space, allowing chemists to predict the chemical behavior of atoms and molecules.
The Heisenberg uncertainty principle states that it is impossible to know both the position and momentum of an electron with infinite precision. This principle has significant implications for our understanding of electron location, as it means that electrons can never be precisely located in space. Instead, their position is described by a probability distribution, which reflects the inherent uncertainty in their location.
Experimental Evidence for Electron Location
Experimental evidence for electron location comes from a variety of sources, including X-ray diffraction, electron microscopy, and spectroscopy. X-ray diffraction, for example, can be used to determine the arrangement of atoms in a crystal lattice, providing information about the location of electrons in the atoms. Electron microscopy, on the other hand, can be used to visualize the electron density around atoms, providing a direct image of the electron location.
Spectroscopy is a powerful tool for studying electron location, as it allows chemists to probe the energy levels of electrons in atoms and molecules. By analyzing the spectra of atoms and molecules, chemists can determine the energy levels of the electrons and gain insight into their location and behavior.
What is the significance of electron location in an atom?
+The location of electrons in an atom determines its chemical properties and behavior, including the type of bonds it can form and its reactivity.
How do chemists determine the electron configuration of an atom?
+Chemists use a variety of techniques, including X-ray diffraction, electron microscopy, and spectroscopy, to determine the electron configuration of an atom.
What is the Heisenberg uncertainty principle, and how does it relate to electron location?
+The Heisenberg uncertainty principle states that it is impossible to know both the position and momentum of an electron with infinite precision, which means that electrons can never be precisely located in space.
In conclusion, the location of electrons in an atom is a complex and fascinating topic that has significant implications for our understanding of chemistry and physics. By understanding the electron configuration of an atom, chemists can predict its chemical behavior and reactivity, and develop new materials and technologies with unique properties.