The XeF4 Lewis structure is a crucial aspect of understanding the molecular geometry and bonding of xenon tetrafluoride. To draw the Lewis structure, we start by determining the total number of valence electrons available. Xenon (Xe) is a noble gas with 8 valence electrons, and each fluorine (F) atom has 7 valence electrons. Since there are four fluorine atoms, the total valence electrons from fluorine are 4 * 7 = 28. Adding the 8 valence electrons from xenon gives a total of 28 + 8 = 36 valence electrons.
Step-by-Step Construction of the XeF4 Lewis Structure
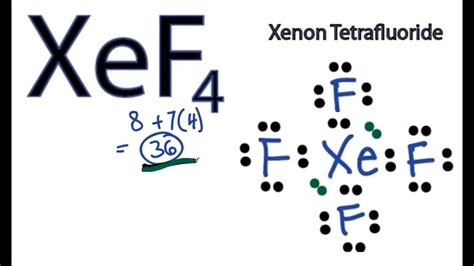
1. Determine the Central Atom: In the XeF4 molecule, xenon is the central atom because it is less electronegative than fluorine. Xenon’s ability to expand its octet due to the availability of d-orbitals for bonding also makes it a suitable central atom.
2. Connect the Atoms: Connect the xenon atom to each of the four fluorine atoms with a single bond. This uses 8 electrons (2 electrons per bond).
3. Satisfy the Octet of Each Atom: After forming the single bonds, xenon has 8 electrons (2 from each of the 4 bonds), and each fluorine has 2 electrons from the bond with xenon. To complete the octet of each fluorine atom, we add 6 more electrons to each fluorine (since each fluorine already has 2 electrons from the bond with xenon, it needs 6 more to reach 8). This leaves us with 36 - 8 (used in bonds) - 24 (added to fluorine) = 4 electrons.
4. Complete Xenon's Octet and Beyond: Xenon already has 8 electrons from the bonds, but we have 4 electrons left. These electrons are added to the xenon atom as two lone pairs, which brings xenon's total electron count to 12 (8 from bonds + 4 as lone pairs). This satisfies the expanded octet of xenon due to its ability to use d-orbitals for bonding and electron accommodation.
XeF4 Molecular Geometry and Polarity
The Lewis structure of XeF4 indicates that the molecule has a square planar geometry. This is due to the arrangement of the four fluorine atoms around the central xenon atom in a plane, with the two lone pairs on xenon situated above and below this plane. The square planar geometry is a result of the VSEPR (Valence Shell Electron Pair Repulsion) theory, where the electron pairs (both bonding and lone pairs) around the central atom arrange themselves to minimize repulsion.
Despite having a symmetrical square planar geometry, XeF4 is a non-polar molecule. This is because the dipole moments of the Xe-F bonds cancel each other out due to the molecule's symmetry, resulting in a net dipole moment of zero.
| Molecular Parameter | Value |
|---|---|
| Molecular Geometry | Square Planar |
| Polarity | Non-polar |
| Bond Angle | 90 degrees |
| Bond Length (Xe-F) | Approximately 1.95 Å |
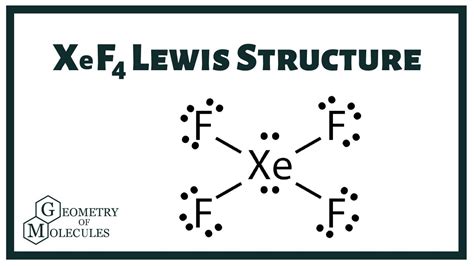
Key Points
- The XeF4 Lewis structure consists of a central xenon atom bonded to four fluorine atoms with single bonds, and two lone pairs on xenon.
- The molecule adopts a square planar geometry due to the arrangement of electron pairs around the xenon atom.
- Despite its symmetrical geometry, XeF4 is non-polar because the dipole moments of the Xe-F bonds cancel each other out.
- Xenon's ability to expand its octet and form bonds with fluorine is crucial for the formation and stability of XeF4.
- The XeF4 molecule is an example of a compound that exhibits an expanded octet, highlighting the versatility of noble gases in forming chemical bonds under certain conditions.
The study of the XeF4 Lewis structure and its molecular geometry not only enhances our understanding of chemical bonding and the periodic table but also underscores the importance of considering the unique properties of elements, such as the ability of certain atoms to expand their octets, in predicting and understanding chemical behavior.
What is the significance of the XeF4 molecule in chemistry?
+XeF4 is significant because it is one of the first compounds discovered that involves a noble gas, challenging the initial belief that noble gases were inert and could not form chemical bonds. Its discovery and study have expanded our understanding of chemical bonding and the properties of noble gases.
How does the XeF4 molecule maintain its stability?
+The stability of XeF4 is attributed to the strong covalent bonds between xenon and fluorine, as well as the molecule’s square planar geometry, which minimizes electron pair repulsions. The ability of xenon to expand its octet by utilizing d-orbitals also contributes to the molecule’s stability.
Is XeF4 used in any practical applications?
+XeF4 has been explored for various applications, including etching in semiconductor manufacturing and as a precursor for other xenon compounds. However, its use is limited due to its reactivity and the challenges associated with handling and storing it.