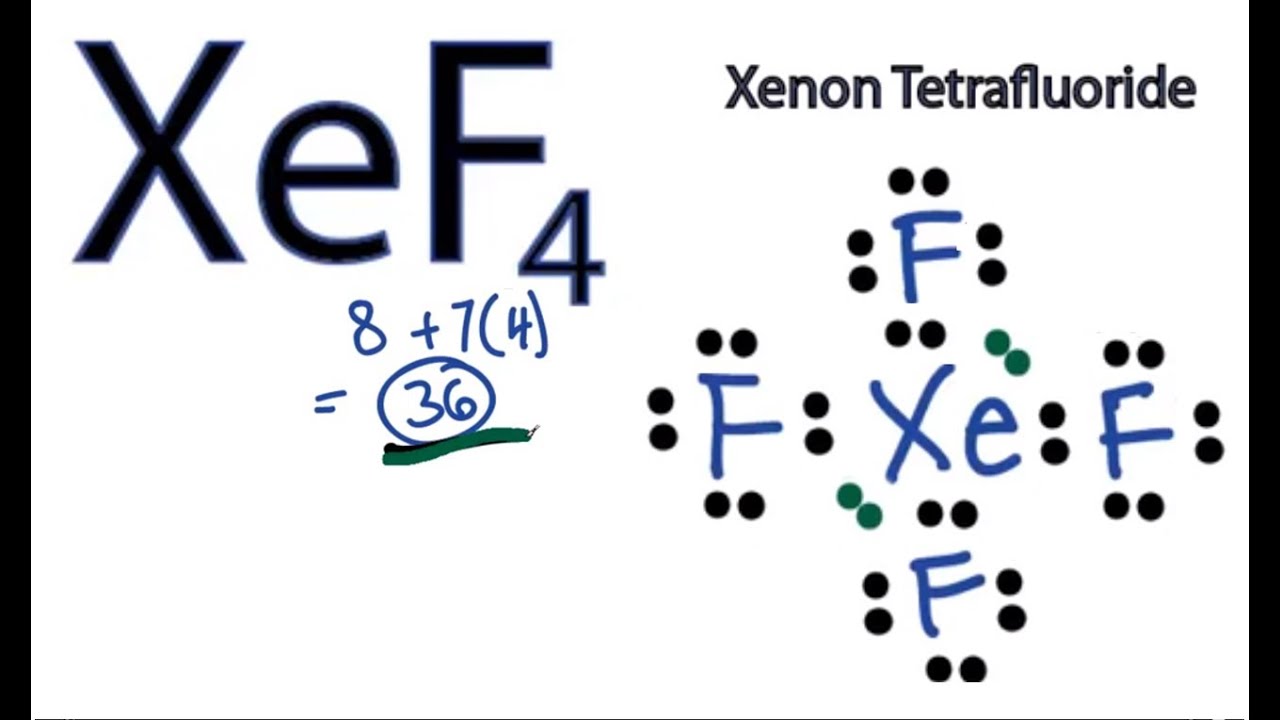
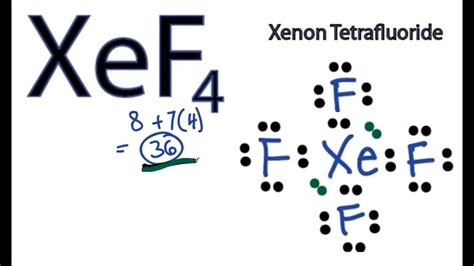
Xenon tetrafluoride, with the chemical formula XeF4, is a compound that has garnered significant attention due to its unique properties and applications. Understanding its Lewis structure is crucial for comprehending its chemical behavior. The Lewis structure, also known as the electron dot structure, is a diagram that represents the bonding between atoms of a molecule and the lone pairs of electrons that may exist. For XeF4, constructing the Lewis structure involves several steps and considerations, reflecting the molecule's geometry and the nature of its chemical bonds.
Naturally Worded Primary Topic Section with Semantic Relevance
To begin with, xenon (Xe) is a noble gas, and fluorine (F) is a halogen. Typically, noble gases are chemically inert due to their full outer shell of electrons. However, under certain conditions, xenon can form compounds with highly reactive elements like fluorine. The Lewis structure of XeF4 can be approached by first calculating the total number of valence electrons available for bonding. Xenon has 8 valence electrons, and each fluorine atom has 7 valence electrons. Since there are four fluorine atoms, the total valence electrons from fluorine are 4 * 7 = 28. Adding xenon’s 8 valence electrons gives a total of 36 valence electrons.
Specific Subtopic with Natural Language Phrasing
The next step in drawing the Lewis structure involves determining the central atom and arranging the other atoms around it. In the case of XeF4, xenon is the central atom because it is less electronegative than fluorine. The four fluorine atoms are then arranged around the xenon atom. Each fluorine atom shares one pair of electrons with xenon in a covalent bond, which accounts for 8 of the valence electrons (2 electrons per bond * 4 bonds). The remaining 28 valence electrons are distributed as lone pairs on the fluorine atoms, with each fluorine having 3 lone pairs (6 electrons), totaling 24 electrons. This leaves 4 electrons, which are placed on the xenon atom as two lone pairs, satisfying the octet rule for all atoms involved.
| Atom | Valence Electrons | Bonding Electrons | Lone Pair Electrons |
|---|---|---|---|
| Xenon (Xe) | 8 | 8 | 4 |
| Fluorine (F) | 28 | 8 | 20 |
| Total | 36 | 8 | 24 |
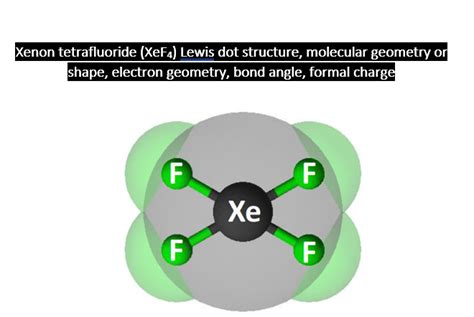
Understanding the Geometry and Polarity of XeF4
The geometry of XeF4 is square planar, with the xenon atom at the center and the four fluorine atoms at the corners of a square. This geometry arises from the octahedral arrangement of electron pairs around the xenon atom, where the two lone pairs occupy positions opposite each other, resulting in a square planar shape for the molecule. This geometry is consistent with the VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts the shapes of molecules based on the number of bonding and lone pairs of electrons around the central atom.
Implications of Molecular Geometry
The square planar geometry of XeF4 has significant implications for its chemical properties. Despite having polar bonds (Xe-F), the molecule is nonpolar due to its symmetrical geometry. The dipole moments of the individual Xe-F bonds cancel each other out, resulting in a molecule with no net dipole moment. This nonpolarity influences the physical properties of XeF4, such as its solubility and boiling point, and its chemical reactivity, including its ability to participate in certain types of reactions.
Key Points
- Xenon tetrafluoride (XeF4) has a unique Lewis structure with a square planar geometry.
- The molecule's stability and reactivity can be understood through its electron pair arrangement.
- XeF4 is nonpolar due to the symmetrical arrangement of its polar Xe-F bonds.
- The molecule's geometry and polarity significantly influence its physical and chemical properties.
- Understanding the Lewis structure and geometry of XeF4 is essential for predicting its behavior in various chemical contexts.
In conclusion, the Lewis structure of XeF4 provides valuable insights into the molecule's geometry, polarity, and chemical behavior. By analyzing the arrangement of electron pairs and the resulting molecular shape, one can predict and understand various aspects of XeF4's chemistry, from its reactivity to its physical properties. This knowledge is not only fundamental to understanding xenon tetrafluoride itself but also contributes to the broader field of inorganic chemistry, particularly in the study of noble gas compounds.
What is the significance of the Lewis structure in understanding XeF4?
+The Lewis structure is crucial for understanding the geometry, polarity, and chemical reactivity of XeF4. It helps in visualizing the arrangement of electrons and predicting the molecule’s behavior in different chemical contexts.
Why does XeF4 have a square planar geometry?
+XeF4 adopts a square planar geometry due to the arrangement of its electron pairs. The two lone pairs on the xenon atom occupy positions opposite each other, leading to a square planar shape that minimizes repulsions between electron pairs.
Is XeF4 a polar or nonpolar molecule?
+Despite having polar Xe-F bonds, XeF4 is a nonpolar molecule due to its symmetrical square planar geometry. The dipole moments of the individual bonds cancel each other out, resulting in no net dipole moment for the molecule.