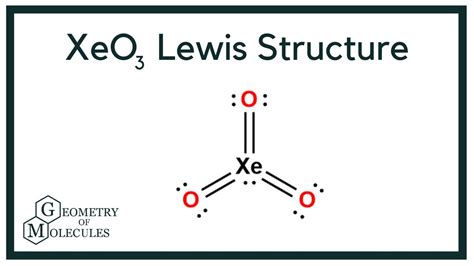
Xeo3, or xenon trioxide, is a compound that has garnered significant attention due to its unique properties and reactivity. Understanding the Lewis structure of Xeo3 is crucial for grasping its chemical behavior and applications. The Lewis structure, also known as the electron dot structure, is a diagram that represents the valence electrons of an atom and the covalent bonds it forms with other atoms.
Key Points
- The Xeo3 molecule has a trigonal pyramidal shape, with xenon as the central atom and three oxygen atoms bonded to it.
- Xenon, being a noble gas, expands its octet in Xeo3 to accommodate more than eight electrons due to the high electronegativity of oxygen.
- The Lewis structure of Xeo3 involves xenon forming three covalent bonds with oxygen, with one of the oxygens having a double bond to xenon.
- Xeo3 is a highly reactive and explosive compound, requiring careful handling and storage.
- Understanding the Lewis structure is essential for predicting the reactivity and properties of Xeo3 in various chemical reactions.
Naturally Worded Primary Topic Section with Semantic Relevance
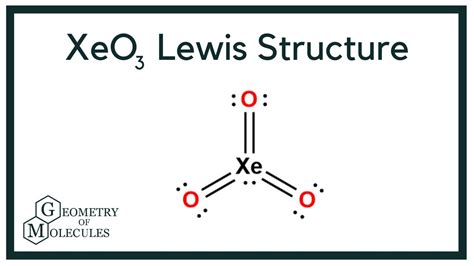
The Lewis structure of Xeo3 can be drawn by following a set of steps that include determining the total number of valence electrons, drawing the skeletal structure, and distributing the electrons to satisfy the octet rule for each atom. Xenon, as a noble gas, typically has a full outer shell with eight electrons, but in the case of Xeo3, it expands its octet to form bonds with the highly electronegative oxygen atoms. This expansion is possible due to the ability of xenon to form an expanded octet, allowing it to accommodate more than eight electrons.
Specific Subtopic with Natural Language Phrasing
The actual process of drawing the Lewis structure for Xeo3 involves starting with the xenon atom as the central atom, to which three oxygen atoms are bonded. Each oxygen atom is assigned two electrons (as a lone pair) initially, and then the remaining electrons are distributed to form single bonds between xenon and each oxygen. Given that xenon has six valence electrons and each oxygen has six, the total valence electrons in Xeo3 are 6 (from xenon) + 3*6 (from the three oxygens) = 24 electrons. After forming three single bonds (using 6 electrons), there are 18 electrons left. These are distributed as lone pairs around the oxygen atoms and the xenon atom, with one oxygen typically forming a double bond with xenon to satisfy the octet rule for all atoms involved.
| Atomic Component | Valence Electrons | Rrole in Xeo3 Lewis Structure |
|---|---|---|
| Xenon (Xe) | 6 | Central atom, expands octet |
| Oxygen (O) | 6 | Bonded to xenon, typically one with a double bond |
Drawing the Lewis Structure
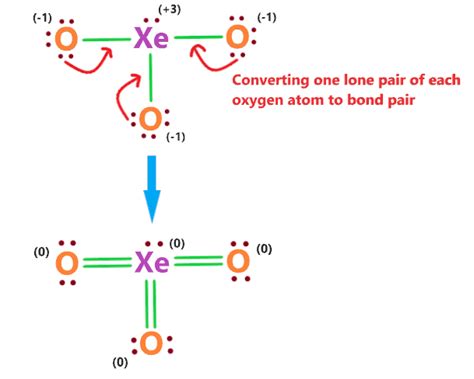
To draw the Lewis structure of Xeo3 accurately, one must consider the total number of valence electrons and the tendency of atoms to achieve a stable electron configuration. The steps include calculating the total valence electrons, sketching the skeletal structure, distributing the electrons to form bonds, and ensuring that each atom (except for hydrogen, which is not present in Xeo3) has an octet. The xenon atom, being less electronegative than oxygen, is the central atom, and it forms bonds with the oxygen atoms to distribute the electrons effectively.
Considering Electronegativity and Octet Expansion
The high electronegativity of oxygen atoms compared to xenon necessitates the expansion of xenon’s octet to accommodate the additional electrons. This expansion allows xenon to form stable bonds with the oxygen atoms, despite initially having a full outer shell. The double bond formation between one oxygen and xenon not only satisfies the octet rule for both atoms but also reflects the higher electronegativity of oxygen, which pulls the shared electrons closer to itself.
Why does xenon expand its octet in Xeo3?
+Xenon expands its octet in Xeo3 due to the high electronegativity of the oxygen atoms, which necessitates the accommodation of additional electrons to form stable bonds.
What is the shape of the Xeo3 molecule?
+The Xeo3 molecule has a trigonal pyramidal shape, resulting from the xenon atom being bonded to three oxygen atoms and having one lone pair.
Is Xeo3 a stable compound?
+Xeo3 is highly reactive and explosive, requiring careful handling. Its stability is limited due to the strained bonds and high reactivity of the xenon-oxygen bonds.
In conclusion, understanding the Lewis structure of Xeo3 is pivotal for comprehending its chemical behavior and reactivity. The unique ability of xenon to expand its octet and form bonds with highly electronegative oxygen atoms underscores the complex and fascinating world of noble gas chemistry. By recognizing the structural aspects and the electron distribution within Xeo3, researchers and scientists can better predict its interactions and applications in various chemical contexts.