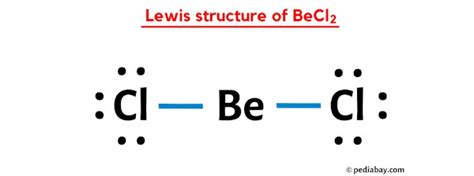
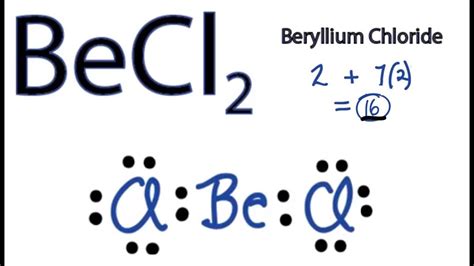The BeCl2 Lewis structure is a fundamental concept in chemistry, representing the molecular geometry and bonding of beryllium dichloride. To understand this structure, it's essential to start with the basics of Lewis structures and the properties of the atoms involved.
Introduction to Lewis Structures
Lewis structures, also known as electron dot diagrams, are a way to represent the valence electrons in a molecule. They help in understanding the bonding and molecular geometry of compounds. The structure is drawn by placing the atoms relative to each other, indicating the bonds between them, and distributing the valence electrons as dots around the atoms.
Understanding BeCl2
Beryllium dichloride (BeCl2) is a chemical compound that consists of one beryllium atom and two chlorine atoms. Beryllium is in group 2 of the periodic table and has two valence electrons, while chlorine is in group 17 with seven valence electrons. The difference in electronegativity between beryllium and chlorine leads to a polar covalent bond.
Drawing the BeCl2 Lewis Structure

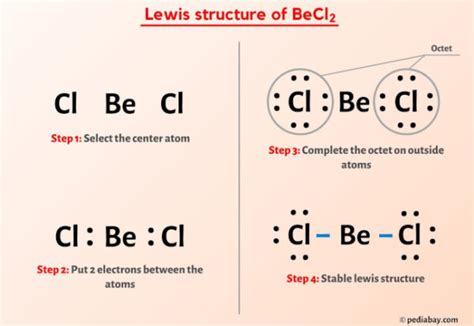
To draw the Lewis structure of BeCl2, follow these steps:
- Place the beryllium atom in the center since it is the least electronegative atom.
- Arrange the two chlorine atoms on either side of the beryllium atom.
- Determine the total valence electrons: Beryllium has 2 valence electrons, and each chlorine has 7, so 2 + (2 * 7) = 16 valence electrons.
- Draw single bonds between the beryllium and each chlorine atom, which accounts for 4 electrons (2 pairs).
- Distribute the remaining electrons (16 - 4 = 12 electrons) around the chlorine atoms to fulfill the octet rule for each chlorine, resulting in three pairs of electrons (6 electrons) on each chlorine atom.
Interpreting the BeCl2 Lewis Structure
The resulting Lewis structure shows a linear geometry for the BeCl2 molecule, with the beryllium atom bonded to two chlorine atoms. This linearity is due to the sp hybridization of the beryllium atom, which results in two equivalent hybrid orbitals directed 180 degrees apart. The bond length between beryllium and chlorine is approximately 1.75 Å, and the Be-Cl bond is polar due to the difference in electronegativity between beryllium and chlorine.
| Atom | Valence Electrons | Electronegativity |
|---|---|---|
| Beryllium (Be) | 2 | 1.57 |
| Chlorine (Cl) | 7 | 3.16 |
Conclusion and Implications
Understanding the Lewis structure of BeCl2 provides insights into its molecular geometry, bonding, and reactivity. The polar nature of the Be-Cl bonds and the linear geometry are critical in understanding the compound’s physical and chemical properties. This knowledge is essential in various chemical and industrial applications where BeCl2 is used.
Key Points
- The BeCl2 Lewis structure represents a linear molecule with a beryllium atom bonded to two chlorine atoms.
- The molecule's geometry results from the sp hybridization of the beryllium atom.
- The Be-Cl bonds are polar covalent due to the electronegativity difference between beryllium and chlorine.
- Understanding the Lewis structure of BeCl2 is crucial for predicting its physical and chemical properties.
- The compound's properties make it useful in various chemical applications and industries.
What is the molecular geometry of BeCl2?
+The molecular geometry of BeCl2 is linear, resulting from the sp hybridization of the beryllium atom.
Why are the Be-Cl bonds in BeCl2 polar?
+The Be-Cl bonds are polar due to the difference in electronegativity between beryllium (1.57) and chlorine (3.16), leading to a partial positive charge on beryllium and a partial negative charge on chlorine.
Meta Description: Learn about the BeCl2 Lewis structure, including how to draw it, its molecular geometry, and the nature of its bonds, to understand the properties and applications of beryllium dichloride.