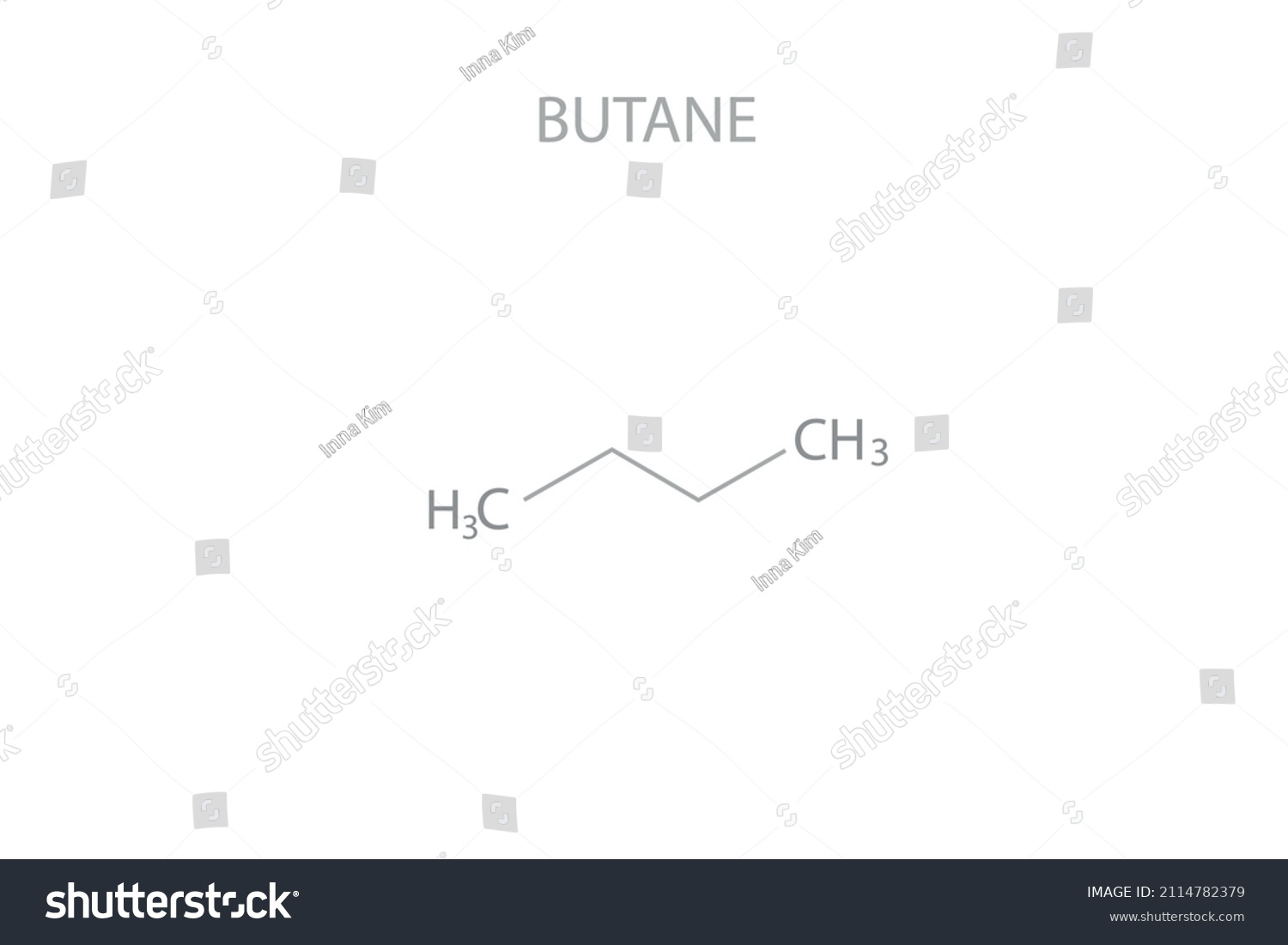
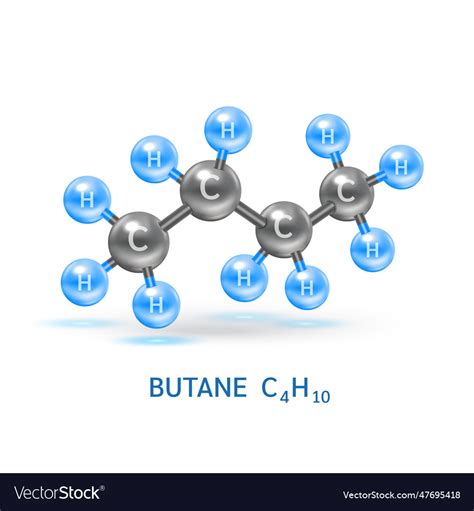
The chemical formula for butane is C4H10, indicating that one molecule of butane consists of four carbon atoms and ten hydrogen atoms. Butane is a hydrocarbon, belonging to the alkane series, and is commonly used as a fuel, an intermediate in the production of other chemicals, and as a refrigerant. Its chemical structure can be represented in two main isomeric forms: n-butane and isobutane. The distinction between these two forms lies in the arrangement of the carbon atoms: n-butane has its carbon atoms arranged in a straight chain, while isobutane has a branched structure. Understanding the formula and structure of butane is crucial for appreciating its physical and chemical properties, including its boiling point, flammability, and reactivity.
Naturally worded primary topic section with semantic relevance
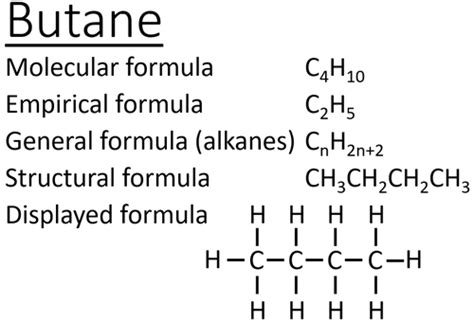
Delving deeper into the properties of butane, it becomes evident that its molecular formula plays a significant role in determining its applications. For instance, the combustion of butane is highly exothermic, releasing a significant amount of energy, which makes it an efficient fuel for cooking, heating, and as a fuel for vehicles. The balanced chemical equation for the combustion of butane is 2C4H10 + 13O2 → 8CO2 + 10H2O, demonstrating the complete oxidation of butane into carbon dioxide and water. Furthermore, the energy density of butane, approximately 45.7 megajoules per kilogram, is a critical factor in its widespread use. This combination of energy release and relatively safe handling characteristics underlines the importance of butane in various sectors.
Specific subtopic with natural language phrasing
A crucial aspect of butane’s application is its phase behavior, particularly its boiling point, which is around -0.5°C for n-butane and -11.7°C for isobutane at standard atmospheric pressure. This property makes butane liquids at room temperature under pressure, facilitating its storage and transportation in cylinders and tanks. Moreover, the critical point of butane, where the distinction between the liquid and vapor phases disappears, is approximately 38.6 bar and 152°C for n-butane. Understanding these physical properties is essential for the design of equipment and systems that handle butane, ensuring safe and efficient operation.
| Property | n-Butane | Isobutane |
|---|---|---|
| Boiling Point (°C) | -0.5 | -11.7 |
| Critical Pressure (bar) | 38.6 | 36.4 |
| Critical Temperature (°C) | 152 | 134.7 |
| Energy Density (MJ/kg) | 45.7 | 45.7 |
Key Points
- The chemical formula of butane is C4H10, indicating four carbon atoms and ten hydrogen atoms per molecule.
- Butane exists in two main isomeric forms: n-butane and isobutane, which differ in their carbon atom arrangement.
- The combustion of butane is highly exothermic, making it a useful fuel.
- The boiling point of butane is critical for its storage and transportation, with n-butane boiling at around -0.5°C and isobutane at -11.7°C.
- Understanding the physical and chemical properties of butane, including its energy density and critical points, is essential for its safe and efficient use.
Butane's versatility and the specific characteristics of its isomers make it a vital component in various industries. From its use as a fuel in vehicles and for cooking, to its application in the production of chemicals and as a refrigerant, the properties dictated by its molecular formula play a pivotal role. The importance of butane in meeting energy demands and its role in chemical synthesis underscores the need for continued research into its properties and applications, ensuring its safe and efficient use.
Industrial Applications and Safety Considerations
Given the widespread use of butane, its handling and storage are subject to strict safety regulations to mitigate risks associated with its flammability and potential for explosion. The storage of butane in well-ventilated areas, away from ignition sources, and the use of appropriate personal protective equipment (PPE) are crucial for preventing accidents. Moreover, the transportation of butane, whether by road, rail, or sea, is governed by specific regulations to ensure public safety. The correct labeling of containers, adherence to quantity limits for transportation, and the training of personnel in emergency procedures are all critical components of butane safety management.
Environmental Impact and Future Directions
The environmental impact of butane, particularly in terms of its contribution to greenhouse gas emissions and its potential for soil and water contamination, necessitates careful consideration. While butane itself is not a greenhouse gas, its combustion produces carbon dioxide, a potent greenhouse gas. Moreover, leaks from butane containers or pipelines can lead to the release of this volatile organic compound (VOC) into the atmosphere, contributing to air pollution. As the world transitions towards cleaner energy sources, the role of butane may evolve, with potential applications in emerging technologies such as fuel cells or as a feedstock for the production of biofuels.
What are the main uses of butane?
+Butane is primarily used as a fuel for cooking and heating, as an intermediate in the production of other chemicals, and as a refrigerant.
What is the difference between n-butane and isobutane?
+The main difference lies in the arrangement of the carbon atoms: n-butane has a straight chain, while isobutane has a branched structure. This difference affects their boiling points and vapor pressures.
Is butane safe to use?
+Butane is generally safe when handled and stored properly. However, it is flammable and can be hazardous if not used in well-ventilated areas or if leaks occur. Following safety guidelines and regulations is crucial for safe use.
In conclusion, butane, with its molecular formula of C4H10, is a versatile and widely used hydrocarbon. Its applications, ranging from fuel to refrigeration, underscore its importance in modern society. However, its use must be balanced with considerations of safety and environmental impact. As technology evolves, the role of butane may adapt, potentially leading to new applications that leverage its unique properties while minimizing its drawbacks. Through continued research and adherence to safety protocols, the benefits of butane can be maximized while its risks are mitigated.