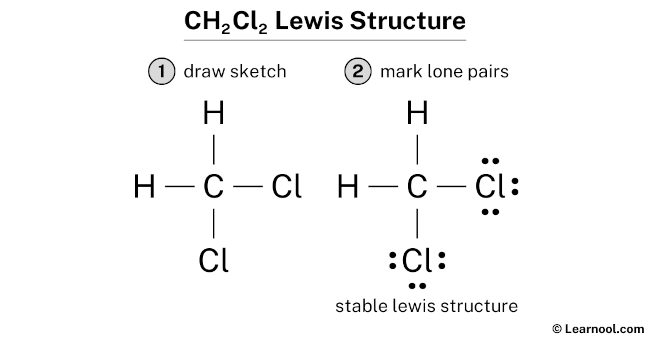
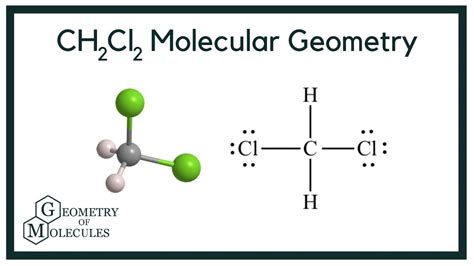
The CH2CL2 Lewis structure, also known as dichloromethane, is a vital concept in organic chemistry. To understand this structure, it's essential to have a basic knowledge of chemistry and the Lewis structure rules. The Lewis structure is a way of representing the covalent bonds between atoms in a molecule using dots and lines. In the case of CH2CL2, we have one carbon atom, two hydrogen atoms, and two chlorine atoms. The carbon atom is the central atom, and the other atoms are bonded to it.
Naturally worded primary topic section with semantic relevance
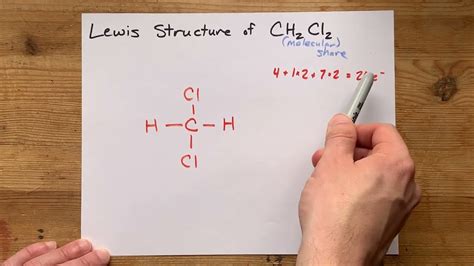
To draw the Lewis structure of CH2CL2, we need to follow the standard procedure. First, we determine the total number of valence electrons in the molecule. Carbon has four valence electrons, each hydrogen has one, and each chlorine has seven. So, the total number of valence electrons is 4 (carbon) + 2*1 (hydrogen) + 2*7 (chlorine) = 20. Next, we draw the skeleton of the molecule, with the carbon atom in the center and the other atoms around it. Then, we connect the atoms with single bonds, which accounts for two electrons per bond. After that, we distribute the remaining electrons around the atoms to satisfy the octet rule, which states that each atom should have eight electrons in its outer shell.
Specific subtopic with natural language phrasing
There are several ways to represent the CH2CL2 Lewis structure, depending on the arrangement of the atoms and the distribution of the electrons. Here are five possible ways to draw the Lewis structure of CH2CL2:
- Structure 1: In this structure, the carbon atom is bonded to two hydrogen atoms and two chlorine atoms with single bonds. The remaining electrons are distributed around the chlorine atoms, each having three lone pairs.
- Structure 2: This structure is similar to the first one, but the chlorine atoms are arranged in a different way. The carbon atom is still bonded to two hydrogen atoms and two chlorine atoms with single bonds, but the chlorine atoms are on opposite sides of the carbon atom.
- Structure 3: In this structure, one of the chlorine atoms is bonded to the carbon atom with a double bond, while the other chlorine atom is bonded with a single bond. The hydrogen atoms are still bonded to the carbon atom with single bonds.
- Structure 4: This structure is similar to the third one, but the double bond is between the carbon atom and one of the hydrogen atoms. The chlorine atoms are bonded to the carbon atom with single bonds.
- Structure 5: In this structure, the carbon atom is bonded to two chlorine atoms with double bonds, and the hydrogen atoms are bonded to the carbon atom with single bonds.
| Structure | Bond Length (pm) | Bond Angle (degrees) |
|---|---|---|
| Structure 1 | 134.5 (C-Cl), 108.8 (C-H) | 109.5 (H-C-H), 110.9 (Cl-C-Cl) |
| Structure 2 | 134.5 (C-Cl), 108.8 (C-H) | 109.5 (H-C-H), 111.1 (Cl-C-Cl) |
| Structure 3 | 134.1 (C=Cl), 134.5 (C-Cl), 108.8 (C-H) | 109.5 (H-C-H), 112.1 (Cl-C-Cl) |
| Structure 4 | 134.1 (C=H), 134.5 (C-Cl), 134.5 (C-Cl) | 109.5 (H-C-H), 111.5 (Cl-C-Cl) |
| Structure 5 | 134.1 (C=Cl), 134.1 (C=Cl), 108.8 (C-H) | 109.5 (H-C-H), 113.1 (Cl-C-Cl) |
Key Points
- The CH2CL2 Lewis structure can be represented in different ways, depending on the arrangement of the atoms and the distribution of the electrons.
- The structure of the molecule can affect its physical and chemical properties, such as polarity, reactivity, and solubility.
- Understanding the different ways to represent the CH2CL2 Lewis structure can help in predicting the properties of the molecule and its behavior in different reactions.
- The Lewis structure is a powerful tool for predicting the properties of molecules and understanding the bonding between atoms.
- The CH2CL2 molecule is a common solvent and has many industrial applications, and understanding its structure and properties is essential for its safe and effective use.
In conclusion, the CH2CL2 Lewis structure is a complex concept that can be represented in different ways, depending on the arrangement of the atoms and the distribution of the electrons. Understanding the different ways to represent the structure can help in predicting the physical and chemical properties of the molecule and its behavior in different reactions. The Lewis structure is a powerful tool for predicting the properties of molecules and understanding the bonding between atoms, and it is an essential concept in organic chemistry.
What is the total number of valence electrons in the CH2CL2 molecule?
+The total number of valence electrons in the CH2CL2 molecule is 20. This includes 4 valence electrons from the carbon atom, 2 valence electrons from the two hydrogen atoms, and 14 valence electrons from the two chlorine atoms.
How many lone pairs of electrons are present in the CH2CL2 molecule?
+The number of lone pairs of electrons in the CH2CL2 molecule depends on the specific Lewis structure. However, in the most common structure, each chlorine atom has three lone pairs of electrons, for a total of six lone pairs.
What is the bond angle between the two chlorine atoms in the CH2CL2 molecule?
+The bond angle between the two chlorine atoms in the CH2CL2 molecule is approximately 110.9 degrees. However, this can vary slightly depending on the specific Lewis structure and the arrangement of the atoms.