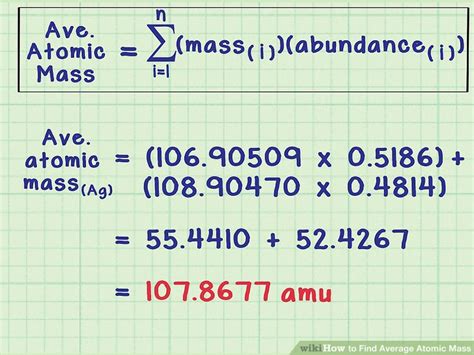
Calculating the average atomic mass of an element is a fundamental concept in chemistry, essential for understanding the properties and behavior of elements. The average atomic mass is a weighted average of the masses of the naturally occurring isotopes of an element. In this article, we will delve into the process of calculating average atomic mass, exploring the underlying principles, formulas, and practical applications.
Key Points
- Understanding the concept of isotopes and their masses
- Learning the formula for calculating average atomic mass
- Applying the formula to real-world examples
- Exploring the significance of average atomic mass in chemistry
- Mastering the calculation of average atomic mass for any element
Understanding Isotopes and Their Masses
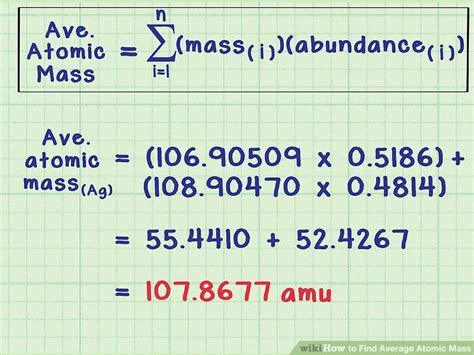
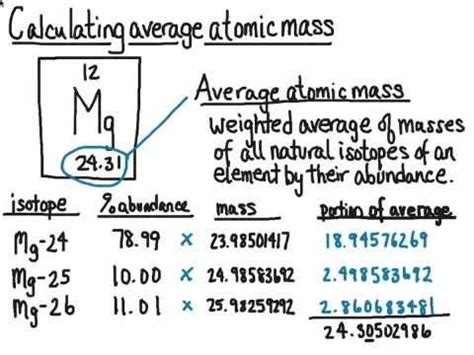
Isotopes are atoms of the same element that have the same number of protons (atomic number) but differ in the number of neutrons, resulting in varying masses. The mass of an isotope is typically expressed in units of atomic mass units (amu) or u (unified atomic mass units). To calculate the average atomic mass, we need to know the masses of the naturally occurring isotopes and their relative abundances.
Formula for Calculating Average Atomic Mass
The formula for calculating the average atomic mass (A_avg) is given by:
A_avg = (m1 \* %abundance1 + m2 \* %abundance2 +... + mn \* %abundancen) / 100
where m1, m2,..., mn are the masses of the isotopes, and %abundance1, %abundance2,..., %abundancen are their respective relative abundances.
| Isotope | Mass (u) | Relative Abundance (%) |
|---|---|---|
| Carbon-12 | 12.000000 | 98.93 |
| Carbon-13 | 13.003355 | 1.07 |
| Carbon-14 | 14.003241 | 0.000000000000001 |
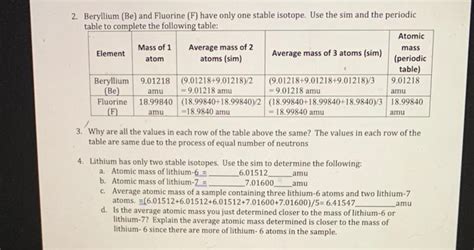
Using the formula, we can calculate the average atomic mass of carbon as follows:
A_avg = (12.000000 \* 98.93 + 13.003355 \* 1.07 + 14.003241 \* 0.000000000000001) / 100 = 12.011
Practical Applications and Significance
The average atomic mass is a critical concept in chemistry, with numerous practical applications. It is used to calculate the molar mass of compounds, determine the number of moles of a substance, and predict the properties of elements. Understanding the average atomic mass is also essential for applications in fields like nuclear physics, materials science, and environmental science.
Calculating Average Atomic Mass for Other Elements
To calculate the average atomic mass for other elements, follow these steps:
1. Identify the naturally occurring isotopes of the element and their masses.
2. Determine the relative abundances of the isotopes.
3. Apply the formula for calculating average atomic mass, using the masses and relative abundances of the isotopes.
By following these steps and using the formula, you can easily calculate the average atomic mass for any element, gaining a deeper understanding of the underlying principles and practical applications of this fundamental concept in chemistry.
What is the significance of average atomic mass in chemistry?
+The average atomic mass is essential for calculating the molar mass of compounds, determining the number of moles of a substance, and predicting the properties of elements. It has numerous practical applications in fields like nuclear physics, materials science, and environmental science.
How do I calculate the average atomic mass for an element with multiple isotopes?
+To calculate the average atomic mass, identify the naturally occurring isotopes and their masses, determine their relative abundances, and apply the formula: A_avg = (m1 \* %abundance1 + m2 \* %abundance2 +... + mn \* %abundancen) / 100.
What is the difference between atomic mass and average atomic mass?
+Atomic mass refers to the mass of a single atom, while average atomic mass is a weighted average of the masses of the naturally occurring isotopes of an element. The average atomic mass takes into account the relative abundances of the isotopes, providing a more accurate representation of the element's composition.
By mastering the calculation of average atomic mass, you’ll gain a deeper understanding of the fundamental principles of chemistry and be better equipped to tackle complex problems in various fields. Remember to always consider the relative abundances of the isotopes and apply the formula to ensure accurate calculations.