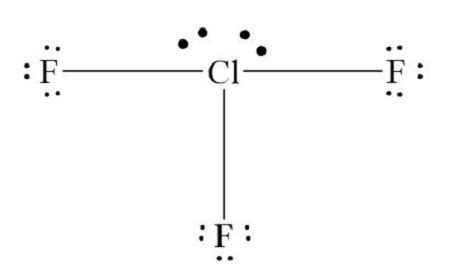
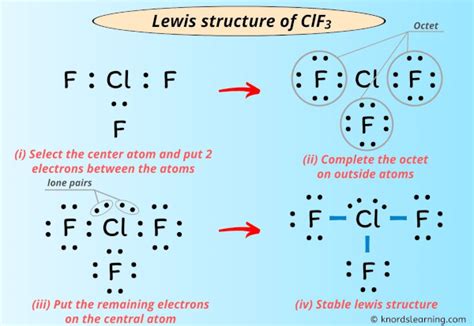
Drawling ClF3, or chlorine trifluoride, requires a basic understanding of chemistry and the structure of molecules. ClF3 is an interhalogen compound, consisting of one chlorine atom bonded to three fluorine atoms. Here's a step-by-step guide on how to draw ClF3 using different methods:
Method 1: Using Basic Valence Shell Electron Pair Repulsion (VSEPR) Theory
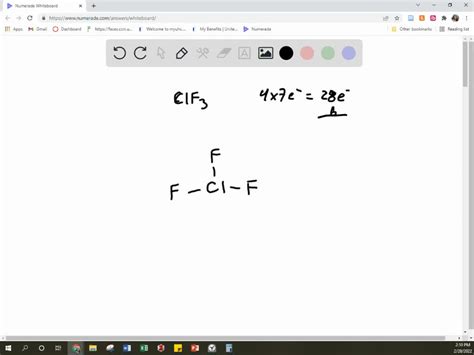
To draw ClF3 using VSEPR theory, start by determining the central atom, which in this case is chlorine (Cl). Chlorine has 7 valence electrons, and each fluorine (F) has 7 valence electrons as well. Since ClF3 has a total of 4 atoms, you’ll have a central chlorine atom bonded to three fluorine atoms. The chlorine atom will have 3 bonding pairs and 2 lone pairs, resulting in a T-shaped molecular geometry.
Step-by-Step Drawing
1. Start by drawing the central chlorine atom as a large circle or dot, representing the nucleus and the inner electrons.
2. Draw three lines extending from the chlorine atom, each representing a bond to a fluorine atom. These lines should be evenly spaced and pointing towards the fluorine atoms.
3. Draw three smaller circles or dots at the end of each line, representing the fluorine atoms.
4. Add 3 pairs of dots between the chlorine and each fluorine atom, representing the covalent bonds.
5. Finally, add 2 pairs of dots to the chlorine atom, representing the lone pairs.
| Molecular Geometry | Description |
|---|---|
| T-shaped | Resulting from 3 bonding pairs and 2 lone pairs around the central chlorine atom |
Method 2: Using Lewis Structures
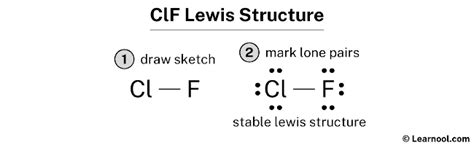
Lewis structures provide a more detailed representation of the molecule, showing the valence electrons and bonds. To draw ClF3 using a Lewis structure, follow these steps:
Step-by-Step Drawing
1. Start by drawing the central chlorine atom, represented by the symbol “Cl”.
2. Draw three fluorine atoms, represented by the symbol “F”, around the chlorine atom.
3. Connect the chlorine atom to each fluorine atom with a single bond, represented by a line.
4. Distribute the valence electrons around the atoms, making sure each fluorine atom has 8 electrons (an octet) and the chlorine atom has 10 electrons (2 lone pairs and 3 bonding pairs).
5. Adjust the bonds and electrons as needed to satisfy the octet rule for each atom.
Method 3: Using 3D Molecular Models
3D molecular models provide a more visual representation of the molecule, allowing you to see the shape and orientation of the atoms in space. To draw ClF3 using a 3D molecular model, follow these steps:
Step-by-Step Drawing
1. Start by creating a central chlorine atom, represented by a large ball or sphere.
2. Add three fluorine atoms, represented by smaller balls or spheres, around the chlorine atom.
3. Connect the chlorine atom to each fluorine atom with a stick or bond, representing the covalent bond.
4. Arrange the fluorine atoms in a T-shaped configuration, with the chlorine atom at the center.
5. Adjust the model as needed to ensure the correct bond angles and molecular geometry.
Key Points
- ClF3 has a T-shaped molecular geometry due to the presence of 3 bonding pairs and 2 lone pairs around the central chlorine atom.
- The molecule can be represented using different methods, including VSEPR theory, Lewis structures, and 3D molecular models.
- Each method provides a unique perspective on the molecule, highlighting its shape, bonding, and electron distribution.
- Understanding the molecular geometry and bonding of ClF3 is essential for predicting its physical and chemical properties.
- ClF3 is a highly reactive and toxic compound, requiring special handling and safety precautions.
Method 4: Using Molecular Orbitals
Molecular orbitals provide a more advanced representation of the molecule, showing the distribution of electrons in the molecule. To draw ClF3 using molecular orbitals, follow these steps:
Step-by-Step Drawing
1. Start by determining the molecular orbitals of the individual atoms, including the chlorine and fluorine atoms.
2. Combine the atomic orbitals to form molecular orbitals, taking into account the overlap and hybridization of the orbitals.
3. Fill the molecular orbitals with electrons, following the rules of molecular orbital theory.
4. Adjust the molecular orbitals as needed to ensure the correct bond order and molecular geometry.
5. Visualize the resulting molecular orbitals to understand the distribution of electrons in the molecule.
Method 5: Using Computational Chemistry
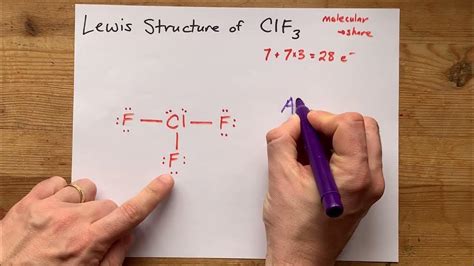
Computational chemistry provides a powerful tool for simulating and visualizing molecules, including ClF3. To draw ClF3 using computational chemistry, follow these steps:
Step-by-Step Drawing
1. Choose a computational chemistry software or program, such as Gaussian or ChemDraw.
2. Input the molecular formula and structure of ClF3, using the software’s built-in tools and libraries.
3. Run the simulation, using methods such as density functional theory (DFT) or Hartree-Fock (HF) to optimize the molecular geometry and predict the physical and chemical properties.
4. Visualize the resulting molecule, using the software’s visualization tools to display the molecular geometry, bond lengths, and electron density.
5. Adjust the simulation as needed, refining the molecular structure and properties to match experimental data or theoretical predictions.
What is the molecular geometry of ClF3?
+The molecular geometry of ClF3 is T-shaped, resulting from the presence of 3 bonding pairs and 2 lone pairs around the central chlorine atom.
How do I draw the Lewis structure of ClF3?
+To draw the Lewis structure of ClF3, start by drawing the central chlorine atom, then add three fluorine atoms around it. Connect the chlorine atom to each fluorine atom with a single bond, and distribute the valence electrons to satisfy the octet rule for each atom.
What are the physical and chemical properties of ClF3?
+ClF3 is a highly reactive and toxic compound, with a strong tendency to react with other molecules and release fluorine gas. It has a high boiling point and a low melting point, and is highly corrosive to metals and other materials.